Figure 2.
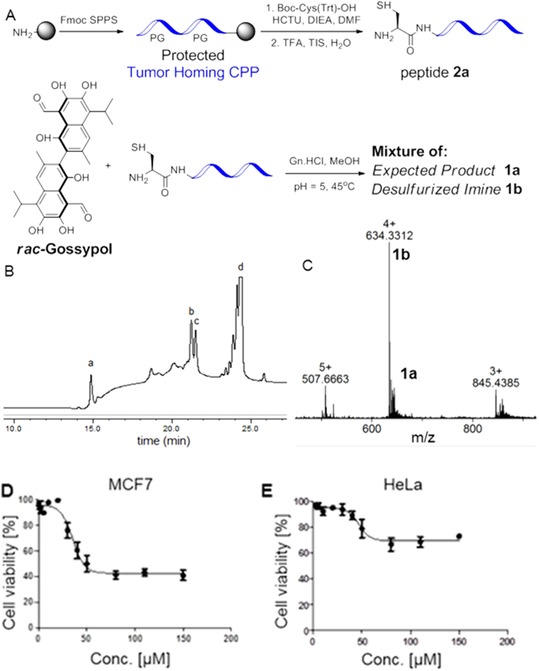
(A) Initial attempt to synthesize the peptide 2 a–gossypol conjugate through thiazolidine ligation led to a mixture from which the active compound 1 b was identified. (B) Analytical HPLC trace of the reaction between peptide 2 a and gossypol at 45 °C after 2 hours with a gradient 5–100 % B in 20 min (buffer B is MeOH with 0.1 % TFA). Peak (I) corresponds to unreacted peptide; peak (II) and (III) correspond to diastereomeric ligation products, whereas peak (IV) corresponds to unreacted gossypol. (C) HRMS data of the ligated product (peak II in the HPLC spectra) after lyophilization. (D) and (E) Cell cytotoxicity assays of MCF‐7 and Hela cells treated with different concentrations of mixture 1 a/1 b isolated from peak (II). MCF‐7 cell (D) and HeLa cell (E) treatment with the conjugation product represented by peak II. Cell viability was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega), the absorptrion values (490 nm) corresponding to each drug concentration were obtained by subtracting the medium only control from all data points and normalizing the absorption to the highest absorption measured in each experiment. Depicted are the average values obtained from three experiments carried out in triplicate. Error bars depicture the standard deviation.
