Table 2.
Aryl Diene Scope in Azadienolate Couplinga
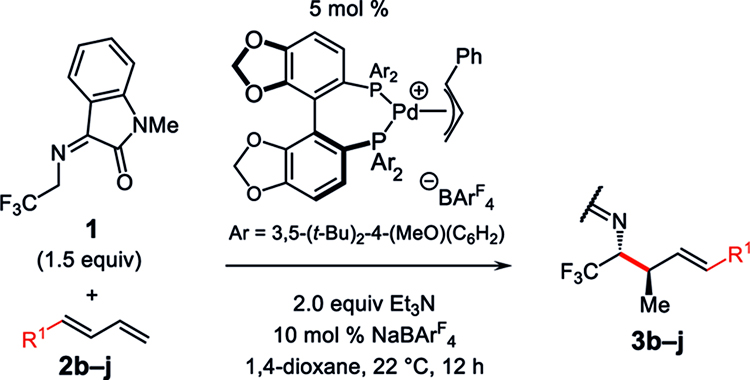 | ||||
|---|---|---|---|---|
| entry | product (3); R1 | conv to 3ab (%); yield of 3c (%) | dr of 3b | er of 3d |
| 1 | 3b; 4-(MeO)(C6H4) | 87; 68 | 10:1 | 94:6 |
| 2 | 3c; 4-CI(C6H4) | 98; 75 | 7:1 | 90.5:9.5 |
| 3 | 3d; 4-(F3C)(C6H4) | 94; 74 | 8:1 | 90:10 |
| 4 | 3e; 3-Me(C6H4) | 89; 72 | 6:1 | 93:7 |
| 5e | 3f; 2-Me(C6H4) | 63; 59 | 2:1 | 92.5:7.5 |
| 6 | 3g; 2-naphthyl | 94; 62 | 8:1 | 94:6 |
| 7 | 3h; 3,4-dioxolato(C6H3) | 94; 86 | 10:1 | 91.5:8.5 |
| 8 | 3i; 2-furyl | 69; 59 | 8:1 | 93:7 |
| 9 | 3j; 3-pyridyl | 83; 82 | 7:1 | 94:6 |
Reactions run under N2 with 0.15 mmol of diene 2 (0.75 M).
Determined by 376 MHz 19F NMR or 400 MHz 1H NMR spectroscopy of the unpurified mixture.
Isolated yield of purified 3.
Determined by HPLC analysis of purified 3.
Reaction run without NaBArF4; 9:1 3f:3f′.
