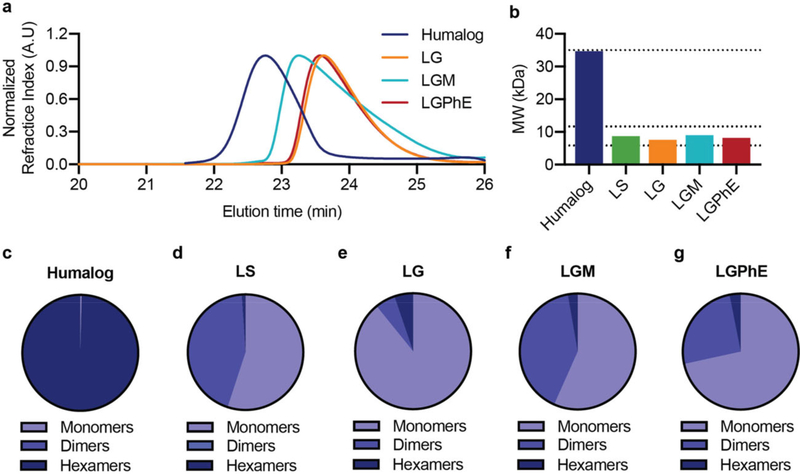
Figure 2.
Association state of lispro with different formulation excipients. Zinc-free insulin lispro association states when formulated in i) phosphate buffer, sodium chloride (0.9%) (LS), ii) phosphate buffer with glycerol (2.6%) (LG), iii) phosphate buffer with glycerol (2.6%) and meta-cresol (0.315%) (LGM), and iv) phosphate buffer with glycerol (2.6%) and phenoxyethanol (0.85%) (LGPhE). Formulations were compared against a formulation of commercial Humalog. a) SEC-MALS elution profiles. b) Number-averaged molecular weight of the distribution of insulin lispro association states. c–g) Ratio of monomers, dimers, and hexamers in each formulation.