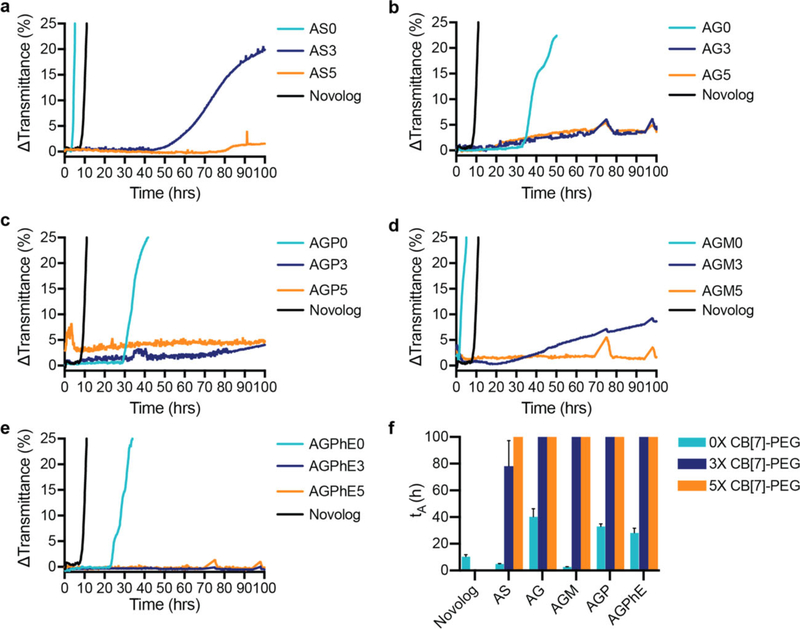
Figure 5.
Formulation with CB[7]-PEG stabilizes zinc-free aspart. In vitro stability of insulin aspart under different formulation conditions with a molar ratio of CB[7]-PEG:Aspart of 0:1 (teal), 3:1 (blue), and 5:1 (orange) against a commercial Novolog control (black). a) Aspart in phosphate buffer with saline (0.9%). b) Aspart in phosphate buffer with glycerol (2.6%). c) Aspart in phosphate buffer with glycerol (2.6%) and phenol (0.25%). d) Aspart in phosphate buffer with glycerol (2.6%) and meta-cresol (0.315%). e) Aspart in phosphate buffer with glycerol (2.6%) and phenoxyethanol (0.85%). f) Comparison of stability by aggregation times (tA), defined as the time to a change in transmittance (λ = 540 nm) of 10% or greater following stressed aging (i.e., continuous agitation at 37 °C). Data shown are average transmittance traces for n =3 samples per group and error bars ((f) only) are standard deviation.