Fig. 3.
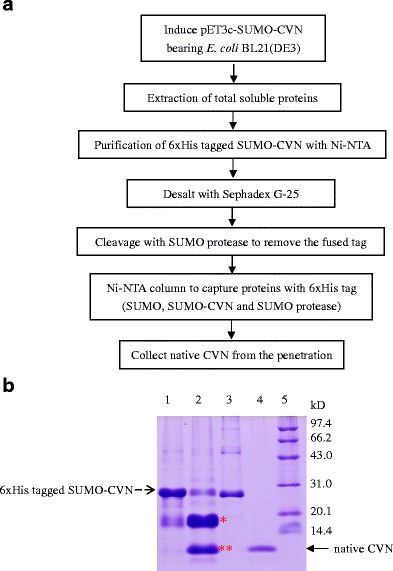
Purification of native CVN from the hexahistidine-tagged SUMO-CVN. a Flow chart of the purification process. Native rCVN was purified by two rounds of Ni2+ chelating chromatography, intervened with one round of hexahistidine-tagged SUMO protease cleavage. b SDS-PAGE analysis of purified rCVN. pET-sumo-cvn bearing E. coli BL21(DE3) was cultured in medium containing 0.5 mM IPTG at 20°C for 24 h. Fractions from the purification were collected and resolved using SDS-PAGE. Lane 1 eluted fraction of the first Ni2+-chelating chromatography; lane 2 SUMO protease cleaved mixture (single asterisk indicates the released hexahistidine-tagged SUMO, double asterisks indicates the rCVN); lane 3 hexahistidine-tagged SUMO protease; lane 4 the eluted fraction of the second Ni2+-chelating chromatography; lane 5 low-molecular-weight protein markers
