Figure 2.
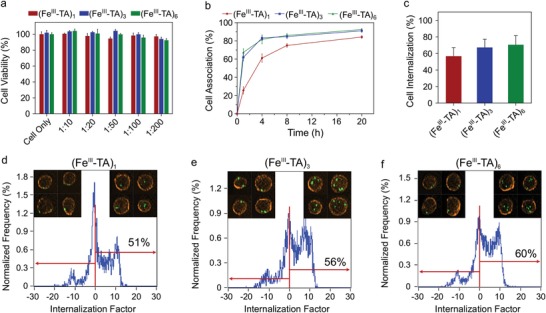
In vitro cell interaction study. a) Cell (A549) cytotoxicity of the (FeIII‐TA)1, (FeIII‐TA)3, and (FeIII‐TA)6 capsules after incubation for 48 h in Dulbecco's modified Eagle's medium with 10% fetal bovine serum at 37 °C at different cell‐to‐capsule ratios. Cell viability was evaluated by the 2,3‐bis[2‐methoxy‐4‐nitro‐5‐sulfophenyl]‐2H‐tetrazolium‐5‐carboxyanilide inner salt (XTT) assay (mean ± SD, n = 4). The viability of untreated cells was normalized to 100%. b) Association of the (FeIII‐TA)1, (FeIII‐TA)3, and (FeIII‐TA)6 capsules with A549 cells over a 20 h incubation period at 37 °C at a cell‐to‐capsule ratio of 1:100. The percentage of cells that associated with the capsules was determined by flow cytometry (mean ± SD, n = 3). c) Internalization of the (FeIII‐TA)1, (FeIII‐TA)3, and (FeIII‐TA)6 capsules in A549 cells after incubation for 20 h at 37 °C at a cell‐to‐capsule ratio of 1:50. The percentage of cells that internalized the capsules was quantified via imaging flow cytometry. No significant differences were observed among (FeIII‐TA)1, (FeIII‐TA)3, and (FeIII‐TA)6 (mean ± SD, n = 3, one‐way ANOVA with Tukey's multiple comparisons test). d–f) Representative images showing the quantification of cell internalization via imaging flow cytometry. The capsules were fluorescently labeled by loading with dextranFITC (green), and the cell membranes were stained with Alexa Fluor 594‐wheat germ agglutinin (orange). The degree of cell uptake was expressed as an internalization factor (IF). The insets show the corresponding representative images of cells with externally surface‐bound capsules (negative IF) and cells with internalized capsules (positive IF).
