Figure 5.
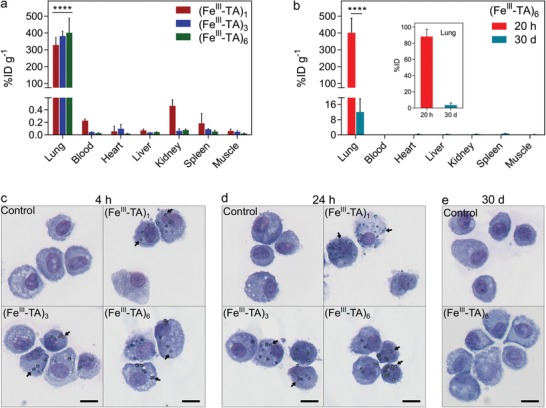
Lung retention and biodegradation study of FeIII‐TA capsules. a) Biodistribution of the (FeIII‐TA)1, (FeIII‐TA)3, and (FeIII‐TA)6 capsules in various organs and blood of mice at 20 h after intratracheal administration. b) Biodistribution of (FeIII‐TA)6 capsules at 20 h and 30 days after intratracheal administration. The data in (a) and (b) are presented as percentage of injected dose per gram of tissue (%ID g−1). The inset in (b) shows percentage injected dose (%ID) of (FeIII‐TA)6 capsules in the whole lung at 20 h and 30 days after administration. All data represent three mice per group. **** in (a) indicates p < 0.0001 for lung versus all other organs, whereas **** in (b) indicates p < 0.0001 for 20 h versus 30 days (two‐way ANOVA with Tukey's multiple comparisons test). c–e) Cytospins of bronchoalveolar lavage cells from lungs of mice at c) 4 h, d) 24 h, and e) 30 days postintratracheal administration of DPBS (control), (FeIII‐TA)1, (FeIII‐TA)3, or (FeIII‐TA)6 capsules. The arrows show examples of capsules that are associated with alveolar macrophages. Scale bars are 10 µm in (c)–(e).
