Figure 7.
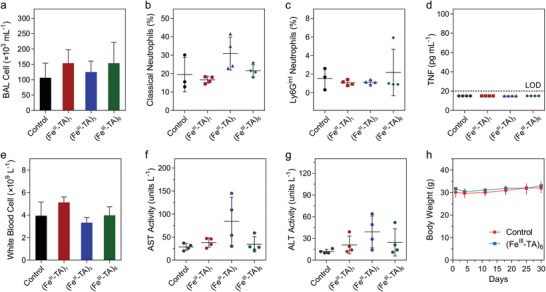
In vivo assessment of inflammation and toxicity of the capsules. Mice were administered intratracheally with either DPBS (control), (FeIII‐TA)1, (FeIII‐TA)3, or (FeIII‐TA)6 capsules (1 × 107 capsules in 100 µL DPBS) and samples were collected 24 h after administration. a) Total number of bronchoalveolar lavage (BAL) cells from lungs of mice. b,c) Relative percentage of both classical and Ly6Gint neutrophils with respect to the total cell yield in whole lungs of mice. d) Concentration of TNF in BAL fluid of mice was below the limit of detection (LOD, 20 pg mL−1) of the assay. e) Concentration of circulating white blood cells from cohorts of mice. f,g) Serum levels of liver enzymes: aspartate aminotransferase (AST) and alanine aminotransferase (ALT). h) Body weight of mice over 30 days after administration. The data in (a)–(h) are shown as mean ± SD (n = 4); no significant differences were seen between control and (FeIII‐TA)1, (FeIII‐TA)3, or (FeIII‐TA)6 groups (one‐way ANOVA with Tukey's multiple comparisons test).
