Figure 4.
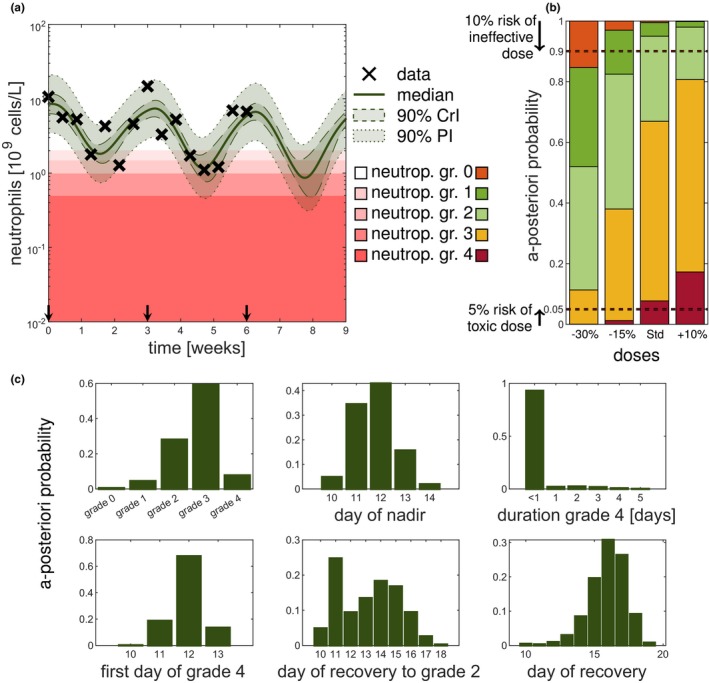
Uncertainty quantification by full Bayesian methods gives important information for therapy dosing selection. The scenario described in multiple cycle study paclitaxel is used and the results are shown for the full Bayes (reference) solution with sampling importance resampling (SIR) using samples. (a) Forecasting the third cycle for different doses based on the patient's covariates and measurements of the first two cycles. (b) Full Bayesian inference allows for probabilistic statements of the different grades. Color coding of neutropenia grades shows trade‐off between efficacy and toxicity. No toxicity (grade 0) is associated with poorer treatment outcome (orange) but severe neutropenia (grade 3 and 4) is also not desired (yellow and red). (c) A posteriori probabilities of quantities of interest for the third cycle based on the posterior at the end of second cycle (week 6) for the standard dose. Statistics, such as day of grade 4, were computed given that grade 4 is reached. Note for all displayed forecasts the full Bayes (reference) approach (SIR with ) was used. CrI, credible interval; PI, prediction interval.
