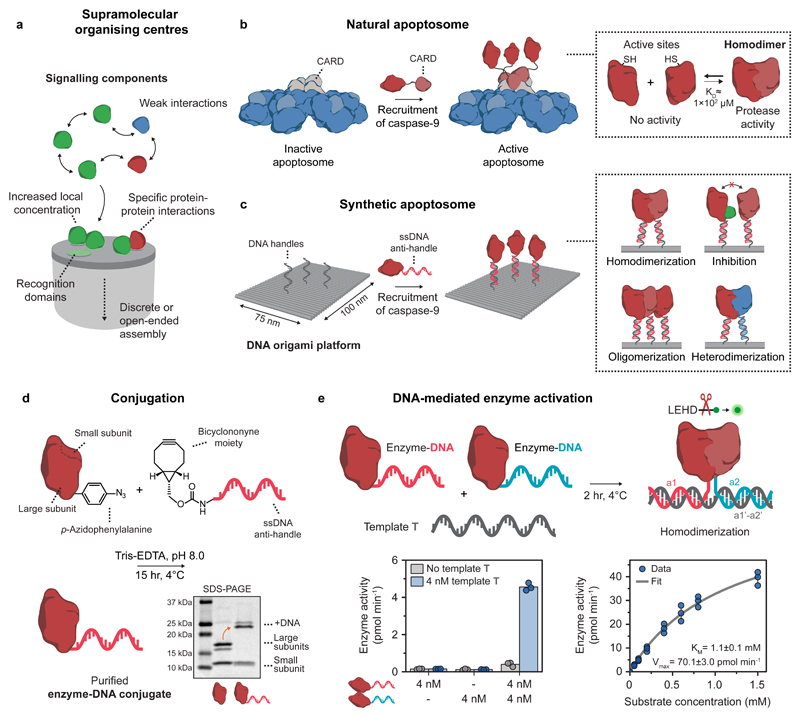
Figure 1. General concept and design elements for the construction of a DNA-based synthetic apoptosome.
a, Schematic concept of supramolecular organising centres (SMOCs). b, Schematic drawing of the natural apoptosome that functions by assembling inactive caspase-9 monomers through caspase recruitment domains (CARDs). The increase in local concentration induces caspase-9 dimerization, leading to proteolytic cleavage of downstream caspases and eventually apoptosis. c, Schematic drawing of the DNA origami-based synthetic apoptosome. Single-stranded DNA (ssDNA) handles on the DNA origami surface recruit caspase-9 monomers with full control over the number, position, and relative geometry, allowing for the characterisation of specific protein-protein interactions such as homodimerization, inhibition, oligomerization, and heterodimerization. d, Reaction scheme for the conjugation of caspase-9 to an oligonucleotide anti-handle. Caspase-9 was expressed in E. coli with unnatural amino acid p-azidophenylalanine at the N-terminus and reacted with a bicyclononyne-functionalized oligonucleotide (8 kDa). Purification with affinity chromatography afforded pure enzyme-DNA conjugates, as shown by SDS-PAGE analysis. Mature caspase-9 consists of a non-covalently bound N-terminal large (18.3 and 19.2 kDa) and C-terminal small subunit (12.8 kDa), which are separated during gel analysis (see also Supplementary Fig. 4). The identity of all protein fragments was confirmed using mass spectrometry analysis (Supplementary Fig. 2). e, Bivalent template T was used to induce dimerization of two identical caspase-9 monomers with different DNA sequences (a1 and a2, indicated in red and blue, respectively). Enzyme activity (left graph) was measured using stoichiometric amounts of all three components and 167 μM of the synthetic caspase substrate LEHD-AFC (Supplementary Fig. 8). Enzyme kinetics (right graph) were determined by measuring activity at 4 nM of each enzyme-DNA conjugate and varying concentrations of the substrate (0-1.5 mM). The data was fitted with the standard Michaelis-Menten expression (see Methods). Bars represent mean enzyme activity. All experiments were performed in independent triplicates.