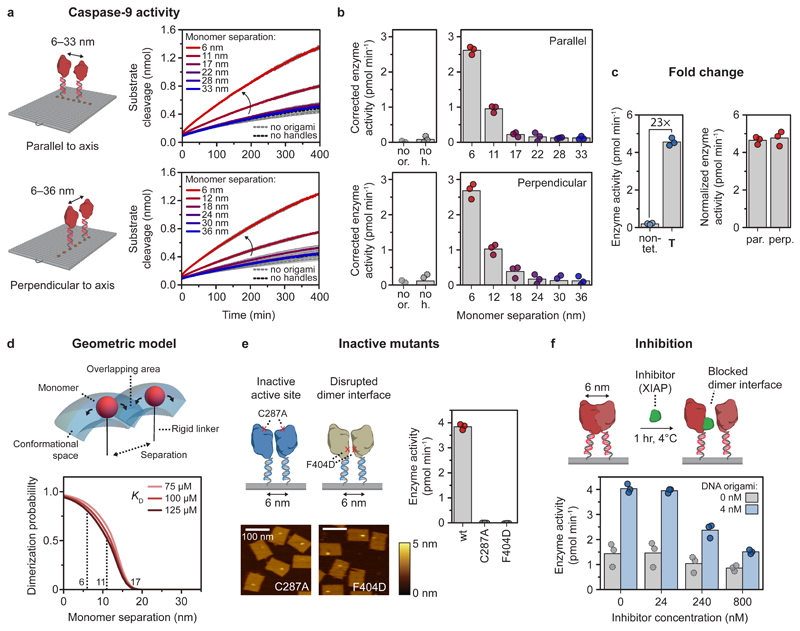
Figure 3. Activation of caspase-9 occurs by distance-dependent dimerization of tethered monomers.
Reactions were carried out with 4 nM DNA origami (unless indicated otherwise) and 3 equivalents of enzyme-DNA conjugate per handle, and incubated for 2 hr at 4°C. Activity was determined by monitoring cleavage of 167 μM LEHD-AFC at 18°C. Bars represent mean activity. All experiments were performed in independent triplicates. a,b, Distance-dependent enzyme activity of two caspase-9 monomers on DNA origami nanostructures parallel (top) and perpendicular (bottom) to the DNA helical axis. Data in a is represented as mean ± s.d. of three independent experiments. Enzyme activity (b) was calculated from the initial slope of the time traces in a and corrected by subtracting the mean background activity (no or.). Labels: no or., no DNA origami present; no h., DNA origami without handles. c, Fold change in enzyme activity for template T, and 6-nm samples parallel (par.) and perpendicular (perp.) to DNA origami helical axis was calculated by comparing with 4 nM non-tethered caspase-9 in buffer (non-tet.). The activity of DNA origami samples was normalized based on an incorporation efficiency per handle of 75%. d, Three-dimensional geometric model assuming free movement for each tethered monomer in the conformational space, determined by molecular dynamics simulations. The tethered dimerization probability was plotted as a function of the KD of caspase-9 dimerization in solution and the separation between the tethered monomers. e, AFM images show correct incorporation of caspase-9 point mutants C287A and F404D on DNA origami nanostructures at 6 nm monomer separation, but both mutants exhibit no enzymatic activity. Colour bars indicate height scale in AFM images. Scale bars, 100 nm. f, Inhibition of caspase-9 by various concentrations of X-linked inhibitor of apoptosis protein (XIAP), in the absence (gray) or presence (blue) of 4 nM 6-nm two-enzyme DNA nanostructures.