Abstract
Cisplatin is used to treat a variety of solid tumors in both children and adults. However, cisplatin has serious side-effects, some of which may permanently affect patients’ quality of life following treatment, such as ototoxicity. There is currently no FDA-approved therapy for the prevention or treatment of cisplatin-induced hearing loss. Herein we examine the potential for statins to prevent cisplatin-induced ototoxicity. Statins, a class of drugs commonly used to prevent or manage hypercholesterolemia, have been of clinical utility for decades with dependable outcomes and reliable safety profiles in humans. Statins are known to be protective in animal models of noise-induced and age-related hearing loss. Moreover, studies have demonstrated an additive benefit of statins in cancer treatment. In the current study, lovastatin reduces cisplatin-induced hearing loss in adult mice. Lovastatin-mediated protection was significantly greater among female than male mice, and the dose of lovastatin required for protection was different between the sexes.
Taken together our data indicate that lovastatin reduces cisplatin-induced hearing loss in mice and suggest that concurrent statin and cisplatin therapy may represent a feasible clinical strategy for reducing cisplatin-induced ototoxicity that should be explored for future clinical use.
Introduction
Cisplatin is a widely used and effective anti-cancer therapy that is used to treat a wide variety of solid tumors (Hohnloser et al., 1995, Helm et al., 2009, Langer et al., 2013, Desari et al., 2014). However, like many traditional chemotherapeutic agents, cisplatin has serious side-effects, some of which may permanently affect patients’ quality of life following treatment. For instance, cisplatin therapy causes significant permanent hearing loss in 20–80% of patients who undergo treatment (Skinner et al., 1990, Blakely et al., 1993, Knight et al., 2005, Coradini et al., 2007, Lewis et al., 2009). The permanence of cisplatin-induced hearing loss greatly impacts quality of life for cancer survivors (Waissbluth et al., 2017, Gentilin et al., 2019). Both children and adults treated with cisplatin often develop a high frequency hearing loss that, compounded by subsequent doses, can progress to severe impairment in frequency regions critical for understanding human speech (Lewis et al., 2009, Theunissen et al., 2015, Schmitt et al., 2018). Such ototoxicity can be dose-limiting for those receiving cisplatin therapy, ultimately affecting survival rates (Kling 2003, Ross et al., 2009, Callejo et al., 2015). With cancer survivorship on the rise (Miller et al., 2016, National Institutes of Health, 2018), quality of life after cancer treatment is a major clinical concern. Therefore, therapies that prevent cisplatin-induced ototoxicity remain an unmet clinical need.
The mechanisms behind cisplatin-induced ototoxicity seem to result from a combination of cisplatin pharmacokinetics in the inner ear and induction of cellular stress. The pharmacokinetic distribution of cisplatin following systemic administration can be tracked throughout the body via detection of its core platinum atom using inductively coupled plasma mass spectrometry (ICP-MS) (Köppen et al., 2015, Breglio et al., 2017). Following systemic administration, cisplatin has been detected in the stria vascularis (SV), organ of Corti, and spiral ganglion neurons (SGN) (van Rujiven et al., 2005, Thomas et al., 2006, Köppen et al., 2015, Chu et al., 2016, Breglio et al., 2017). Dysfunction of any one of these inner ear structures is detrimental to hearing sensitivity and speech understanding. Furthermore, we previously showed that platinum levels increase in the mouse inner ear as early as one hour following systemic cisplatin administration and that cisplatin is retained in both the human and murine inner ear indefinitely (Breglio et al., 2017). Taken together, potential therapies for preventing cisplatin-induced ototoxicity would prevent uptake of the drug in the inner ear tissues and/or combat the toxic cellular stress responses induced by cisplatin.
The induction of heat shock proteins (HSPs) in response to cellular stress is the most ubiquitous stress response in biology (Martindale et al., 2002). In the inner ear, HSP induction is a critical response shown to protect hair cells against major stresses (Taleb et al., 2008, Taleb et al., 2009, Kim et al., 2010, Francis et al., 2011, May et al., 2013, Baker et al., 2015). One of these protective HSPs is heme oxygenase-1 (Hmox1, also known as heat shock protein 32, HSP32), an enzyme that degrades heme into carbon monoxide (CO), biliverdin, and free iron (Maines et al., 1992, Hosick et al., 2017). Induction of Hmox1/HSP32 reduces cisplatin-induced hair cell death in whole-organ cultures of utricles from adult mice (Baker et al., 2015). These data suggest that pharmaceutical induction of Hmox-1 might hold promise as a co-therapy to prevent cisplatin-induced hearing loss.
Statins are a class of drugs used primarily to lower low-density lipoprotein (LDL) cholesterol levels in blood by inhibiting a rate-limiting enzyme, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, in cholesterol synthesis. However, statins exhibit pleiotropic effects, including decreased oxidative stress and inflammation (Liao et al., 2009, Bu et al., 2011) that can yield therapeutic effects beyond reduced serum cholesterol levels. Many statin drug types are known inducers of Hmox1/HSP32 (Lee et al., 2004, Chen et al., 2006, Kwok et al., 2011). Specifically, lovastatin induces Hmox1 expression in the liver, brain, lung, and heart within 24-hr of administration (Hsu et al., 2006, Ali et al., 2009). Statins are Food and Drug Administration (FDA)-approved, and they have excellent safety and low toxicity profiles in humans (Schachter 2004, Hu et al., 2012, Ramkumar et al., 2016). Importantly, statins are not tumor protective (Gazzero et al., 2012) and in fact have demonstrated some anti-tumor activity (Zhong et al., 2015, Deng et al., 2019, Chen et al., 2019, Xia et al., 2019). If statins reduce cisplatin-induced hearing loss, statin co-therapy could be rapidly translated into clinical use to protect the hearing of patients undergoing cisplatin therapy.
The goal of the present study was to determine whether lovastatin could prevent cisplatin-induced hearing loss using a previously described and clinically relevant mouse model of cisplatin-induced ototoxicity (Fernandez et al., 2019). Lovastatin was selected as it is known to cross the blood-brain barrier (Hsu et al., 2006), a feature that may imply its ability to cross the blood-labyrinth barrier. The blood-labyrinth barrier remains a physiologic obstacle to many drugs designed to target the inner ear (Nyberg et al., 2019).
Methods
Animals
Thirty-two 12-week-old CBA/CaJ mice (16 male, 16 female) from the Jackson Laboratory (Bar Harbor, Maine, USA) were randomly assigned to one of six different treatment groups, maintaining balanced sexes across groups: 1) saline control, 2) low-dose lovastatin, only (40 mg/kg, “LVA 40”), 3) high-dose lovastatin, only (60 mg/kg/day, “LVA 60”), 4) cisplatin, only (3 mg/kg/day, “Cis Only”), 5) cisplatin + low-dose lovastatin (“Cis + LVA 40”), or 6) cisplatin + high-dose lovastatin (“Cis + LVA 60”). All mice were housed individually with free access to food and water in accordance with the NIH NIDCD/NINDS Animal Care and Use Committee-approved protocol (#1327).
Mice in cisplatin-treated groups (Cis Only, Cis + LVA 40, Cis + LVA 60) underwent a 3-cycle cisplatin administration protocol as previously described (Fernandez et al., 2019). See Supplementary Data 1 for full details. Within a single cycle, mice received once daily cisplatin injections (3 mg/kg/day, IP, Fresnius Kabi, Lake Zurich, IL) for four consecutive days followed by 10 days of recovery. This cycle was repeated three times for a total of 42 days (cumulative cisplatin dose for all three cycles = 36 mg/kg). Mice in non-cisplatin-treated groups (Saline control, LVA 40, LVA 60) received comparable volumes of sterile saline (0.9% NaCl, IP) on drug injection days. Saline-treated control mice served as a room control to confirm that animals were not acquiring noise-induced or age-related hearing loss over the 6-week cisplatin administration period. All lovastatin-treated mice (LVA 40, LVA 60, Cis + LVA 40, Cis + LVA 60) received once daily doses of lovastatin (Selleck Chemicals, LLC, Houston, TX, USA) via oral gavage (20G, Roboz FN-7910) beginning three days prior to the start of cisplatin administration in addition to daily lovastatin administration throughout the 42-day cisplatin administration period. Cisplatin-only treated mice (Cis Only) were administered the lovastatin vehicle via oral gavage at volumes equivalent to LVA 60 without the lovastatin drug (See Supplementary Data 1 for full detail).
Mice in cisplatin-treated groups received twice daily nutrition and hydration support to maintain body weight and overall health (Fernandez et al., 2019). Each morning, mice received 1 ml of subcutaneous 0.9% normal saline solution and 0.3 ml (per os, PO) STAT® liquid high-calorie supplement (PRN Pharmacal, Pensacola, FL, USA). In the afternoon, mice were given 1 ml of subcutaneous Normasol® saline (Hospira, Inc., Lake Forest, IL, USA). Additional food pellet chow and DietGel® Recovery cups (ClearH2O, Portland, ME, USA) were placed on the cage floor. Mice were carefully monitored by both the study investigators and veterinary staff for changes in health and activity that may have occurred as a result of cisplatin treatment. A body conditioning score (BCS) was assigned daily based on muscular tone, body fat content, coat maintenance, and overall energy level (Ullman-Culleré and Foltz, 1999). Per our animal protocol, any mouse receiving a BCS<2, indicative of severe malnutrition, was to be promptly euthanized; however, no mouse met this criterion. Keeping the cisplatin-treated mice healthy enough to complete the study requires constant monitoring as well as additional nutrition and hydration support. This protocol can only be carried out in adult mice aged at least 11 weeks prior to the onset of cisplatin administration. A detailed protocol for caring for cisplatin-treated animals is included in Supplementary material. For full review of the protocol, see Fernandez et al., 2019. Two mice died due to anesthesia-related deaths; one during baseline auditory testing (prior to cisplatin) and one during the posttest phase. In total, groups consisted of 4 saline control (2M, 2F), 4 LVA 40 (2M, 2F), 3 LVA 60 (2M, 1F), 8 Cis Only (4M, 4F), 6 Cis + LVA 40 (2M, 4F), and 6 Cis + LVA 60 (3M, 3F).
Tests of hearing function
Distortion product otoacoustic emissions (DPOAE), an indirect measure of outer hair cell function, and auditory brainstem response (ABR) thresholds, a measure of hearing sensitivity, were recorded prior to the start of the cisplatin administration protocol in all mice and again after the completion of the third cisplatin administration cycle. All auditory tests were conducted in a sound-attenuated booth (Acoustic Systems, Austin, TX, USA) on mice anesthetized using ketamine (Pulney Inc, Portland, ME, USA; 100 mg/kg, IP) and xylazine (Akorn Inc, Lake Forest, IL, USA; 10 mg/kg, IP) with supplemental injections consisting of 1/3–1/2 of the initial dose, if needed. Core body temperature was maintained at 37°C using a temperature-controlled heating pad fitted to a rectal probe (World Precision Instruments ATC-2000, Sarasota, FL, USA).
DPOAE and ABR measurements were recorded using Tucker-Davis Technologies (TDT; Alachua, FL, USA) hardware (RZ6 Processor) and software (BioSigTZ). For DPOAE tests, a single acoustic assembly consisting of an ER-10B+ (Etymotic, Elk Grove Village, IL, USA) connected to two TDT MF-1 transducers was inserted into the external ear canal such that an unobstructed path from port to tympanic membrane was established with an appropriate acoustic seal. Two primary tones consisting of L1=65 dB SPL and L2 =55 dB SPL were presented at 14 f2 frequencies ranging 4–40 kHz (f2/f1=1.2). The amplitude of the DPOAE at 2f1–f2 was recorded with background noise estimates at 6 surrounding spectra. Immediately following DPOAE recordings, a closed-field TDT MF-1 speaker was placed in the left ear of each mouse, and subdermal needle electrodes (Rhythmlink, Columbia, SC, USA) were placed at the vertex (noninverting), under the test ear (inverting), and at the base of the tail (ground). Tone-burst stimuli (Blackman window, 3 ms, 1.5 ms rise/fall in alternating polarity) were presented at a rate of 29.9/sec at 8, 11.2, 16, 22.4, 32, and 40 kHz starting at 90 dB SPL. The waveforms from 1,024 presentations were averaged, amplified (20x), filtered (0.3–3 kHz) and digitized (25 kHz). Stimulus level decreased in 20 dB steps and in then in 5 dB steps near threshold. Auditory threshold was defined as the lowest stimulus level that elicited a reproduceable waveform with identifiable peaks.
Histology
All mice were euthanized via CO2 inhalation followed by decapitation at the end of the third cycle of cisplatin administration after completion of auditory tests. Immediately after removal of the temporal bones, 4% paraformaldehyde (PFA) at 4° C was perfused through cochlear oval and round windows. Cochleas were fixed overnight in 4% PFA at 4° C and then decalcified for 48 hours at room temperature in 0.5M EDTA (pH 8.0). Once decalcified, one cochlea from each animal was either microdissected or remained whole for measurement of platinum content by inductively-coupled plasma mass spectrometry (ICP-MS). The other cochlea from each mouse was microdissected and immunostained in whole mount.
Decalcified cochlear tissue samples for analysis by ICP-MS were trimmed of all vestibular structures and end organs as well as excess temporal bone fragments. In microdissected samples, the stria vascularis (SV) was removed from the lateral wall using a curved single prong of a #5 forceps (Fine Science Tools) while suspended in UltraPure™ Distilled Water (Invitrogen); organ of Corti and spiral ganglion neuron (SGN) samples were isolated from their surrounding tissues using an ophthalmic straight knife (Accutome). Each region’s sample was the collective amount of stria, organ of Corti or SGN gathered from each cochlear turn from base to apex. Samples were prepared by removing residual liquid via speed vacuum concentration (Eppendorf Vacufuge) and kept frozen at −80° C until ready for submission.
Both whole and microdissected cochlear samples were analyzed by ICP-MS at the University of Amherst Mass Spectrometry Facility. Tissue sample tubes were labeled by a unique identifier void of treatment group information to keep the analyses blinded. Platinum measurements were performed using a NexION 350D inductively coupled plasma-mass spectrometer (Perkin Elmer). The instrument was tuned daily for optimum performance and sensitivity. Whole organs and microdissected samples were dried prior to analysis. Samples were digested by addition of 50 μl of trace metal nitric acid (Fisher Chemical) and incubated for 20 min at 65 °C. An equal volume of hydrogen peroxide (Optima Grade,Fisher Chemical) was added, and the incubation was repeated. Samples were diluted 1:20 with water prior to analysis. A quantitative analysis method was established using dynamic reaction mode (DRC) with oxygen as the reaction gas at 1.2 ml/min flow rate. Platinum and sulfur single element standards (Perkin Elmer) were used to generate standard curves (using 0.5 ppt to 10 ppb standards for Pt and 5 ppb to 2 ppm standards for sulfur). This method was used to quantitatively measure platinum (Pt 195) and sulfur (as SO 48). Platinum values were normalized to sulfur concentration for each sample, since samples were too small to be accurately weighed for normalization.
Cochleas used for immunostained whole mounts were dissected into 5 pieces (Eaton Peabody Laboratories, 2018a), rinsed in 1x PBS, suspended at room temperature for 1 hr in blocking solution comprised of 5% normal horse serum (NHS; Sigma-Aldrich, St. Louis, MO, USA) and Triton X-100 (Sigma-Aldrich; 1:300). Cochlear pieces were immunofluorescently stained with antibodies to 1) C-terminal binding protein 2 (mouse anti-CtBP2; BD Biosciences, San Jose, CA; used at 1:200) and 2) myosin-VIIa (rabbit anti-myosin VIIa; Proteus Biosciences, Ramona, CA; used at 1:200) with secondary antibodies conjugated to Alexa Fluor 568 (Invitrogen; used at 1:1000) and 647 (Invitrogen, used at 1:200), respectively. A cochlear place-frequency map (Müller et al., 2005) was generated using a custom plug-in to ImageJ (Eaton Peabody Laboratories, 2018b) to localize cochlear structures to corresponding frequency regions.
Microscopy
Immunofluorescently stained cochlear turns were imaged at frequency locations (Müller et al., 2005) corresponding to the ABR test frequencies used to assess changes in auditory sensitivity using an LSM 780 laser scanning confocal microscope (Carl Zeiss AG, Oberkochen, Germany). Z-stacks (0.2 μm step size) were collected in a 1024 × 1024 raster (135 μm2) using a high-resolution oil-immersion objective (63x). Inner (IHC) and outer hair cells (OHC) were counted based on their CtBP2-stained nuclei along a 75 μm length of the cochlea for each frequency using Zen Blue software (Carl Zeiss Microscopy GmbH, 2011, v2.3).
Gene Expression Analysis
Nine adult male CBA/CaJ mice were used separately to assess whether lovastatin induced heat shock protein expression within the mouse cochlea. Three mice treated with 60 mg/kg lovastatin and three mice treated with 40 mg/kg/day lovastatin received once daily drug administration via oral gavage for four days. Three mice treated with saline (at an equivalent volume to 60 mg/kg/day lovastatin) served as controls. On the fourth day, mice were euthanized via CO2 inhalation followed by decapitation 3 hours after the last injection. The temporal bones were extracted, and the vestibular system, as well as any excess bone, was quickly removed with a #11 scalpel blade. Both cochleas from each animal were placed in a single 1.5 ml microcentrifuge tube pre-filled with 100ul of RNAlater™ Stabilization Solution (Qiagen, #AM7021) until all samples could be collected for RNA extraction. Total RNA from each sample was extracted using the Qiagen miRNeasy micro plus Kit (Qiagen, #74034) following the manufacturer’s protocol. Genomic DNA was eliminated by on-column DNase digestion. The quality and quantity of the purified RNA was examined by measuring absorbance at 260 nm and 280 nm using a Nanodrop 2000 Spectrophotometer (Nanodrop Technologies, LLC). All samples had 260/280 ratios of ~2.0. To examine the expression level of targeted genes, the purified RNA samples were reverse transcribed using a SuperScript™ VILO™ cDNA Synthesis Kit (Invitrogen, # 11754050). Resulting cDNA was amplified using the TaqMan™ Gene Expression Assay (Applied Biosystems, #4331182) using the StepOnePlus™ Real-Time PCR System (Applied Biosystems, # 4376600). Taqman probes for this study include hspa1a (Mm01159846_s1), hspa1b (Mm03038954_s1), hspb1 (Mm00834384_g1), hspd1 (Mm00849835_g1) hspe1 (Mm07295795_g1), hmox1 (Mm00516005_m1), hsp90aa (Mm00658568_gH), hsp90ab (Mm00833431_g1), and gapdh (Mm99999915_g1).
Following amplification, the Comparative CT (ΔΔCT) method was used to calculate the relative expression of each target gene from 3 biological replicates for each experimental condition. The variation in the relative quantification value was calculated using the standard deviation algorithm based on the mean of the ΔCT and is graphically represented as error bars. The reverse transcription and amplification experiments were each run twice to ensure reproducibility.
Statistics
All statistical analyses were carried out using Graph Pad Prism 8 software. Significant probability values were set to an alpha of <0.05. To compare pre- versus post-treatment ABR data in control and cisplatin-treated mice, a Repeated Measures (RM) Analysis using a mixed-effects model was applied to absolute threshold and amplitude data. In instances where two group comparisons were made, i.e., male versus female, an unpaired, two tailed t-test was used. A two-way analysis of variance (ANOVA) was used to determine main effects of lovastatin treatment across multiple groups. This was applied to ABR threshold shift data, DPOAE post-protocol amplitude data, HC counts, and cisplatin levels measured using ICP-MS. Post hoc multiple comparison analyses controlling for False Discovery Rate (FDR) (Benjamini, Krieger and Yekutieli) were used to determine significance between specific groups.
Results
Lovastatin reduces cisplatin-induced ototoxicity
All mice were subjected to hearing testing prior to cisplatin administration using auditory brainstem response (ABR) and distortion product otoacoustic emission (DPOAE) assays to determine baseline hearing sensitivity and outer hair cell function. Mice underwent a 42-day cisplatin administration protocol (Fig 1A) previously shown to induce a clinically relevant cisplatin lesion (Fernandez et al., 2019). At the end of the cisplatin protocol, ABR and DPOAE measurements were repeated, and cochlea were removed for hair cell quantification and platinum concentration measurements. ABR threshold shifts are reported as the change in threshold between pre- and post-test auditory test sessions. Supplementary Table 1 includes information about group means, standard deviations, and group sizes for each experiment. Control (saline-treated) mice exhibited no significant change, on average ≤ +/−5 dB, in auditory sensitivity relative to baseline (Fig 1B, F1,18=1.846, p>0.05). Similar to controls, mice that received only lovastatin did not show significant changes, again on average ≤ +/− 5 dB, in sensitivity with either 40 mg/kg (F1,18=0.0005, p>0.05) or 60 mg/kg (F1,30=0.2540, p>0.05) dosing (Fig 1B). In contrast, all cisplatin-treated mice had significant increases in ABR thresholds across the frequency range (Fig 1B, F(1,84)=564.8, p<0.001), with greater threshold shifts at higher frequencies, reaching a 49 dB +/− 5.6 threshold shift at 40 kHz. No significant differences between male vs. female mice were observed in the cisplatin lesion (Fig 1C and 1D, t10=0.01914, p>0.05); on average, threshold shift differences ranged from −6.3 to 3.8 dB at each frequency.
Figure 1: Cisplatin ototoxicity is reduced by lovastatin co-therapy.
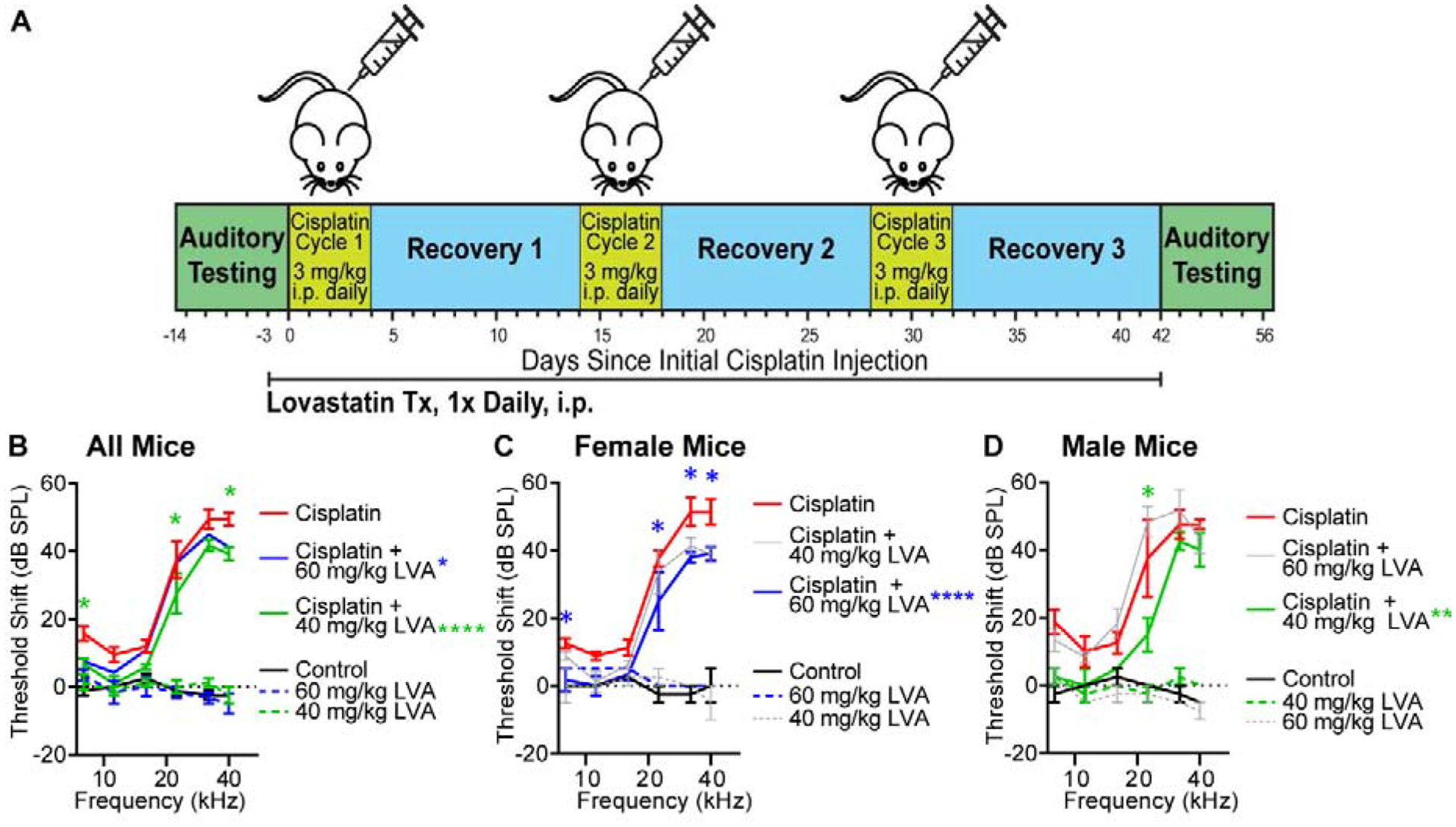
A: Auditory sensitivity was evaluated via auditory brainstem response (ABR) in all mice at baseline and following completion of 3 cycles of cisplatin administration. Threshold shifts are reported as the difference in threshold between pre-cisplatin and post-cisplatin measurements. B: Cisplatin-treated mice (red) demonstrated significant high-frequency threshold elevations relative to saline-treated mice (Panel B). Lovastatin treatment, alone (dashed lines), did not impact auditory thresholds. Overall, mice co-treated with cisplatin and lovastatin had significantly smaller threshold shifts compared to cisplatin alone (Cisplatin+40mg/kg LVA, green, Cisplatin+60mg/kg LVA, blue). C: Female cisplatin-treated mice showed greatest protection when co-treated with 60 mg/kg lovastatin (blue, Panel B). 40 mg/kg lovastatin (grey) was not protective in female mice. D: Cisplatin-treated male mice had maximum protection from 40 mg/kg lovastatin (green, Panel C). Lovastatin was not protective at 60 mg/kg in male mice (grey). Mean ± SEM, n=8 Cisplatin (4M, 4F), n=6 Cisplatin + 60 mg/kg LVA (3M, 3F), n=6 Cisplatin +40 mg/kg LVA (3M, 3F), n=4 Control 92M, 2F), n=3 60 mg/kg LVA (2M,1F), n=4 40 mg/kg LVA (2M, 2F). Statistical analysis consisted of 2-way ANOVA multiple comparisons. *p<0.05, **p<0.001 ****p<0.0001.
Overall, a significant main effect of treatment was observed when comparing the ABR threshold shifts of cisplatin-treated mice against those that received both cisplatin and lovastatin (Fig 1B, F2,102=10.32, p<0.0001). Maximal protection was achieved with 40 mg/kg cisplatin (Cis + LVA 40). Threshold shifts were significantly reduced by an average of 9, 10 and 10 dB +/− 4.66 at 8, 22.4, and 40 kHz (p<0.05), respectively. However, when the protective effect was examined separately in male and female mice, a sex difference was observed (Fig 1C, D). Lovastatin treatment was significantly protective in female mice (Fig 1C, F2,48=15.68, p<0.0001), The greatest protective effect in female mice was observed with 60 mg/kg lovastatin (LVA 60) with a significant difference in ABR threshold shift consisting of 10.8 dB, 13.3 and 12.3 dB +/− 4.93 at 8, 32 and 40 kHz, respectively (p<0.05) (Fig 1B). Lower dose lovastatin (Cis + LVA 40) was also protective in female mice with significant protection at 32 kHz (10 dB +/− 4.577) and 40 kHz (12.5 dB +/− 4.577) (Fig 1C). In male mice, lovastatin was more protective at 40 mg/kg (Fig 1D, F2,36=8.030, p=0.0013), and at this dose significant protection was observed at 22.4kHz with an average 22.5 dB +/− 8.093 lower threshold shift (p<0.01). 60 mg/kg lovastatin was not protective in male mice (Fig 1D).
DPOAEs provide an indirect measure of outer hair cell (OHC) function. At the end of Cycle 3, control (saline-treated) mice and mice that received lovastatin-only (no cisplatin) had no significant reduction in DPOAE amplitudes relative to baseline (F(3,360)=5.702, p<0.001; p>0.05 for all multiple comparisons) (Fig 2A). In contrast, all cisplatin-treated mice showed significant decreases in DPOAE amplitudes relative to baseline (F1,616=700.1, p<0.0001) (Fig 2A), with the greatest reduction at higher frequencies (f2>15.3 kHz, p<0.0002) with an average difference in amplitude as large as 37.2 +/−2.352 at F2= 28.8 kHz. No significant differences were observed between male and female mice in terms of the cisplatin-induced decrease in DPOAE amplitudes (Fig 2A, t26=0.4738, p>0.05).
Figure 2: Cisplatin treatment impairs out hair cell function; lovastatin provides modest protection.
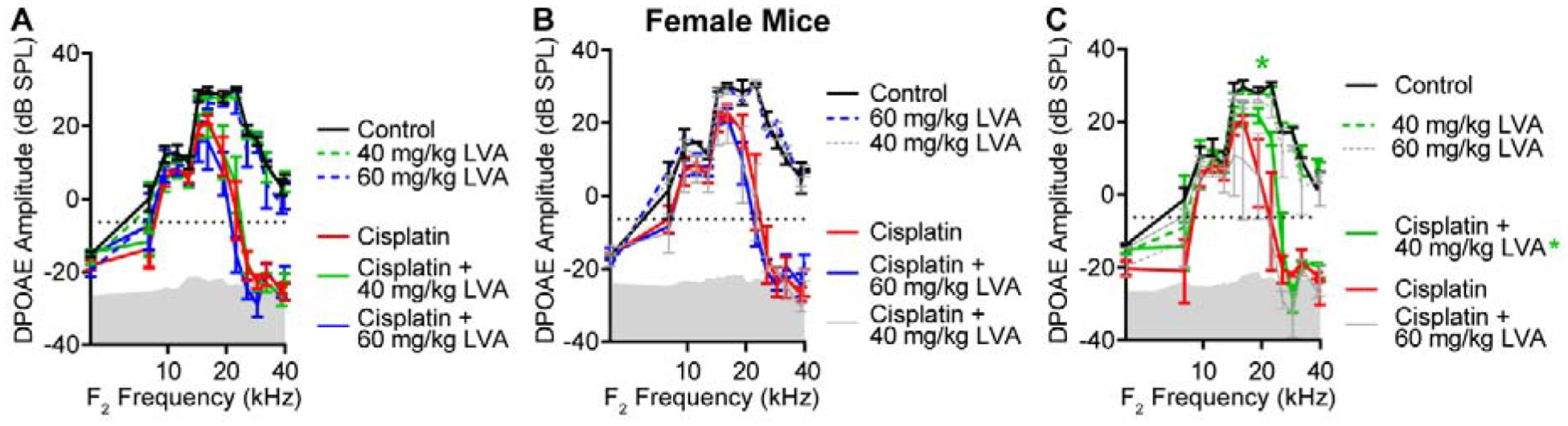
Distortion product otoacoustic emissions (DPOAE) were recorded from mice in all treatment groups. An emission at 2f1–f2 was deemed present when its amplitude was greater than −5 dB (dotted line). Control and lovastatin-treated mice (dashed lines) maintained normal DPOAE amplitudes across an F2 range of 8–40 kHz. Mice treated with cisplatin (solid lines) had significantly reduced DPOAE amplitudes at 10–32 kHz with absent emissions at f2 frequencies above 18 kHz. Lovastatin treatment did not significantly improve DPAOE emission amplitudes after cisplatin when all mice were included in the analysis (A) or in female mice (B). Male mice (C) that received cisplatin plus 40 mg/kg lovastatin had significantly higher DPOAE amplitudes than those that received cisplatin only (red) at f2=17.9 kHz. Mean ± SEM, n=8 Cisplatin (4M, 4F), n=6 Cisplatin + 60 mg/kg LVA (3M, 3F), n=6 Cisplatin +40 mg/kg LVA (3M, 3F), n=4 Control 92M, 2F), n=3 60 mg/kg LVA (2M,1F), n=4 40 mg/kg LVA (2M, 2F). Statistical analysis consisted of 2-way ANOVA with multiple comparisons. *p<0.05. Gray shaded region denotes biological noise floor.
When both sexes were examined together, there was no significant benefit of either 40 or 60 mg/kg lovastatin on DPOAE amplitudes of cisplatin-treated mice (Fig 2A). No main effect of lovastatin treatment on DPAOE amplitudes was observed in female mice (Fig 2B). A significant main effect of lovastatin was observed in cisplatin-treated males that were co-treated with 40 mg/kg lovastatin (Fig 2C, F1,56=4.517, p=0.038). Post hoc analysis using multiple comparisons (Benjamini, Krieger and Yekutieli) indicated significance at f2=17.9 kHz (Fig 2C, p=0.0088) with a mean amplitude difference of 22.76 dB +/− 8.38. Together these data indicate that lovastatin reduces cisplatin-induced hearing loss in mice, and the protective effect of lovastatin varies with both the dose of lovastatin and the sex of the animal.
Lovastatin co-therapy reduces cisplatin-induced cochlear outer hair cell loss
Upon completion of post-Cycle 3 auditory tests, mice were euthanized, and their cochleas were processed for histology. Confocal z-stacks were collected from frequency-specific locations along the basilar membrane (Müller et al., 2005) corresponding to the six ABR frequencies tested. IHCs and OHCs were identified based on the presence of Myosin-VIIa and CtBP2 labeling. As reported previously (Fernandez et al., 2019), cisplatin did not affect IHC survival (F5,60=1.075, p>0.05) (Fig 3, Fig 4A). However, consistent with the loss of auditory sensitivity and DPOAE amplitude detailed above, OHC density was significantly reduced at cochlear locations corresponding to frequencies ≥22.4 kHz in cisplatin-treated mice relative to control mice (Figs 3, 4B). Cisplatin mice co-treated with lovastatin at both 40 and 60 mg/kg showed greater OHC survival in the mid- to high-frequency region with up to 6.8 and 5.5 (+/−2.485) increased OHC survival per 75 μm at 32 kHz, respectively. (2way ANOVA, p<0.04). Taken together with the DPOAE data, these data suggest that the protective effect of lovastatin against cisplatin-induced hearing loss may be partially independent of protection of outer hair cells.
Figure 3: Cisplatin-induced outer hair cell loss is reduced in cisplatin mice co-treated with lovastatin.
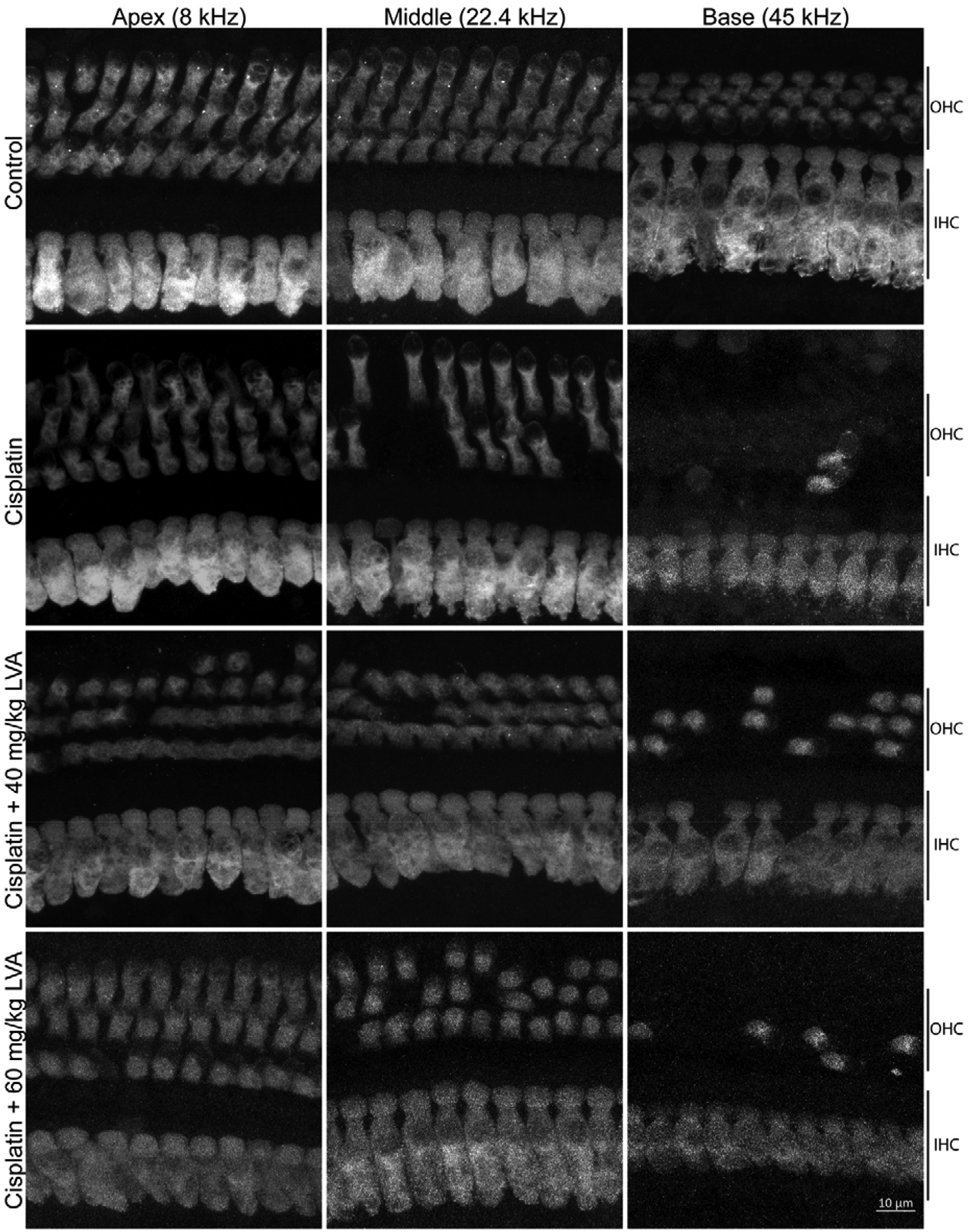
Microdissected cochlear tissue immunofluorescently stained for myosin-VIIa were imaged for the purpose of quantifying inner and outer hair viability following cisplatin treatment. Representative images are shown from the cochlear apex (8 kHz), middle (22.4k Hz) and base (45 kHz) to show the HC densities for the three rows of OHC and single row of IHC for saline-treated control (1st row), cisplatin-treated (2nd row), cisplatin + low dose lovastatin (3rd row), and cisplatin + high dose lovastatin (4th row). See Figure 4 for quantification of each cell type.
Figure 4: Lovastatin reduces high frequency outer hair cell loss in cisplatin-treated mice.
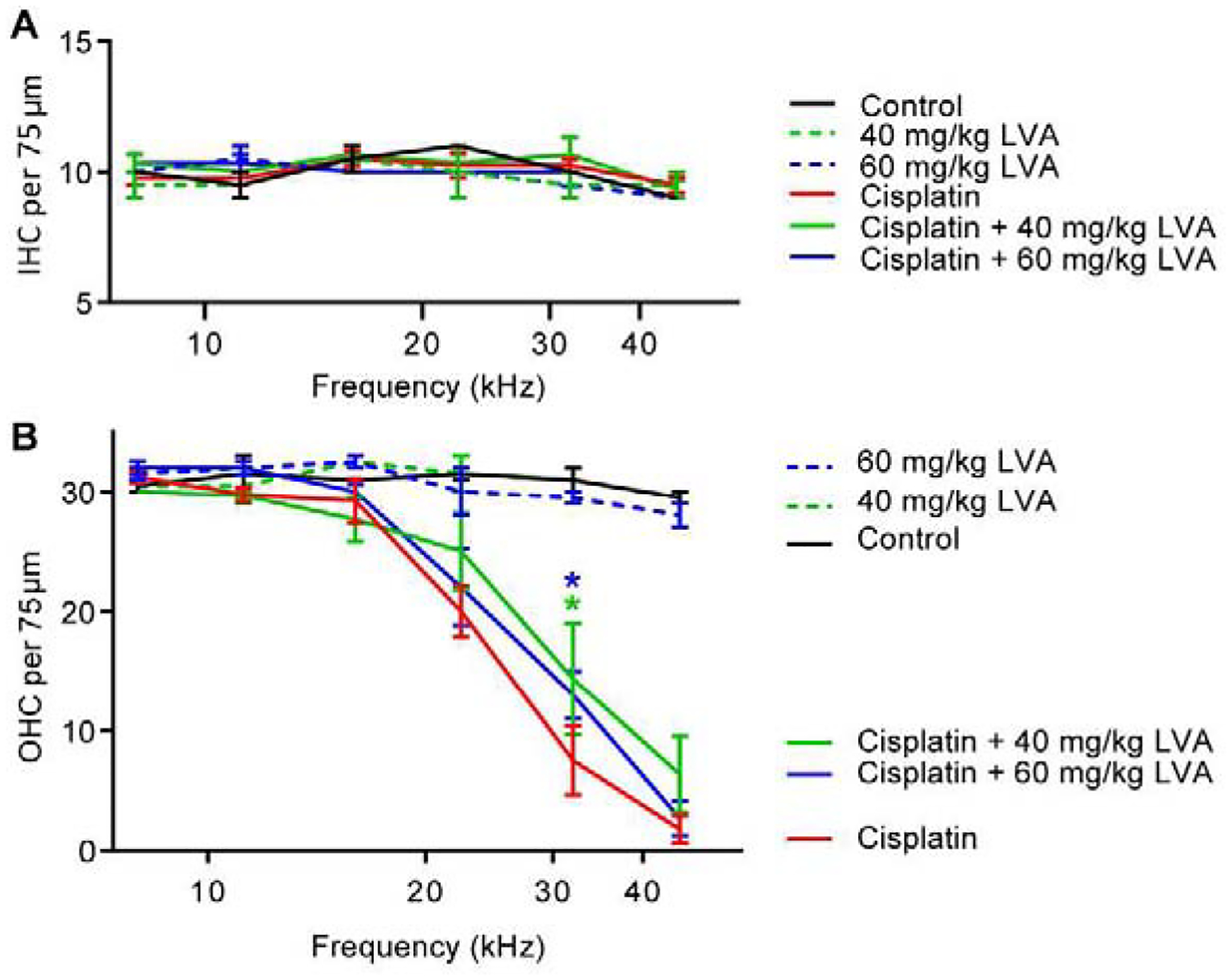
Hair cells were counted in immunofluorescently stained cochlear tissue after the last cycle of cisplatin administration. Hair cells were quantified in the region of 6 discrete frequency locations aligned with ABR test frequencies. Panel A: Inner hair cells (IHC) remained intact in all lovastatin and cisplatin-treated mice. Panel B: Cisplatin-treated mice (red) had significant OHC loss at frequency locations >16 kHz. Lovastatin was protective against cisplatin-induced OHC death in the 32 kHz cochlear region. Mean ± SEM, n=4 Cisplatin, n=3 Cisplatin + 60 mg/kg LVA, n=3 Cisplatin +40 mg/kg LVA, n=2 Control, n=2 60 mg/kg LVA, n=2 40 mg/kg LVA. Statistical analysis consisted of 2-way ANOVA. *p<0.05.
Lovastatin treatment does not reduce platinum entry to the cochlea
Our previous data indicate that cisplatin enters the cochlea and is retained there indefinitely (Breglio et al., 2017). In order to begin to address the mechanisms by which lovastatin reduces cisplatin ototoxicity, we investigated whether lovastatin reduces cisplatin accumulation in the inner ear. Platinum levels were measured by ICP-MS on whole cochleas and microdissected cochlear tissue. Platinum levels were significantly elevated in cisplatin-treated cochlear samples relative to control tissue samples (F3,58=58.03, p<0.001) (Fig 5A). As reported previously (Breglio et al., 2017), platinum accumulation was highest in the stria vascularis followed by the organ of Corti and spiral ganglion neurons (Fig 5A). Lovastatin did not alter platinum levels in whole cochleas or in microdissected organ of Corti, spiral ganglion neurons, or stria vascularis (F2,50=0.1110, p>0.05) from mice of either sex (Figure 5A). These data indicate that the protective effect of lovastatin does not occur via a mechanism of reduced uptake of cisplatin into the inner ear.
Figure 5: Lovastatin does not alter cisplatin entry to the inner ear; it does induce a heat shock response.
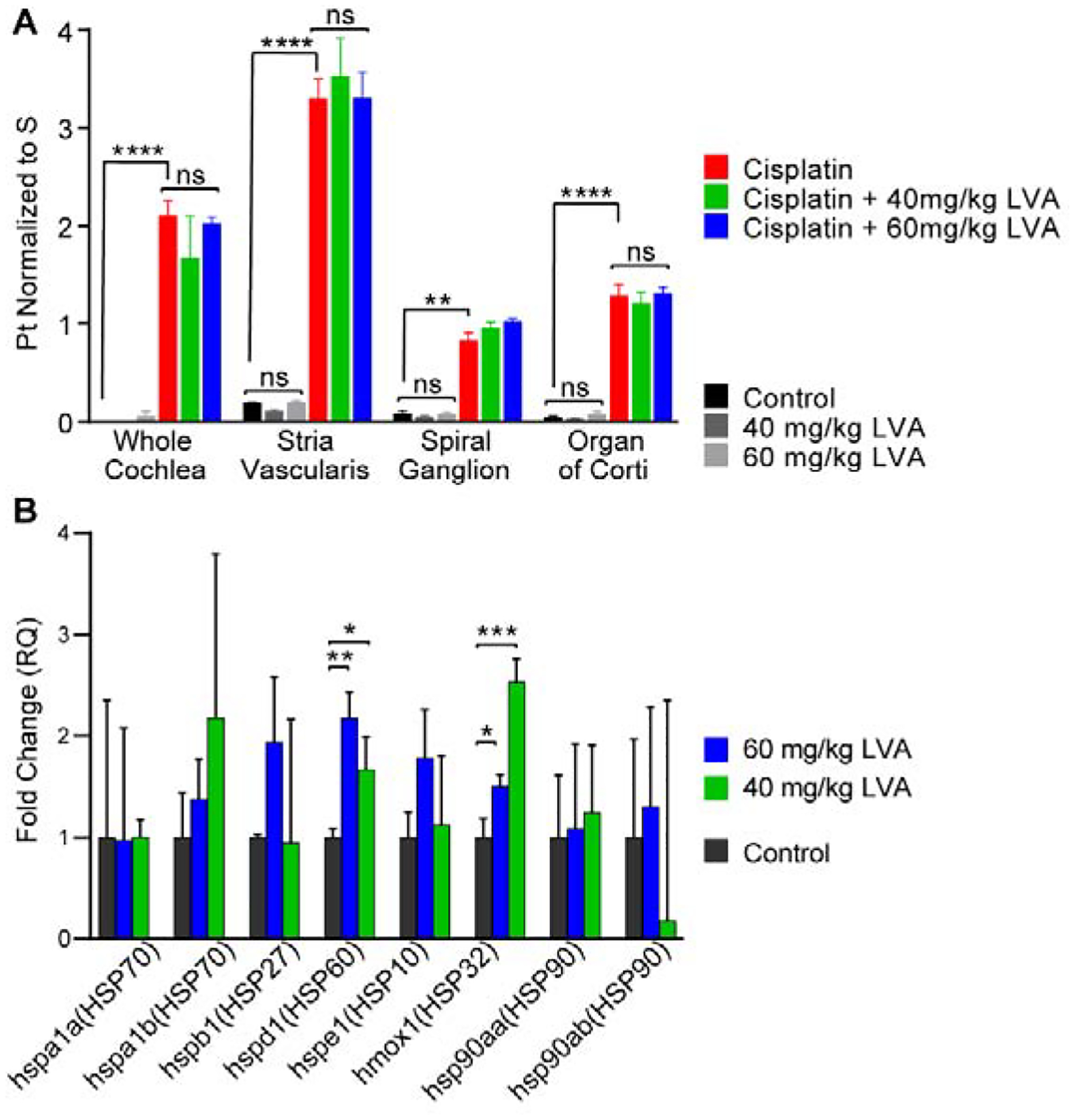
A: Platinum concentrations in whole cochlea samples as well as in microdissected stria vascularis, spiral ganglion, and organ of Corti tissue pieces collected following post-Cycle 3 auditory testing were measured using ICP-MS. Platinum levels were elevated in all tissue sample types in cisplatin-treated mice (red) relative to saline-treated controls (black). Lovastatin (blue, green) did not reduce platinum levels in any of the inner ear tissues. Mean ± SEM, n=3–5 per condition. Statistical analysis consisted of a 2way ANOVA with multiple comparisons. **p<0.01, ****p<0.0001, ns = no significance. B: Lovastatin induced Hmox1 and HSP60 mRNAs in whole cochleas. HSP mRNA levels were measured using qRTPCR. Both 40 and 60 mg/kg lovastatin significantly increased levels of HSP60 and Hmox1 (HSP32) mRNA in the cochlea. Mean ± SD, n=3 biological replicates per condition. Statistical analysis consisted of Multiple t tests. *p<0.05, **p<0.01, ***p<0.001. Lovastatin protects against cisplatin-induced hearing loss in mice
Lovastatin induces Hmox1 in the cochlea
In order to examine whether lovastatin upregulates Hmox1 in the cochlea, nine male CBA/CaJ mice were treated once daily for four days with either saline, 40 mg/kg lovastatin, or 60 mg/kg lovastatin. Three hours after the final drug treatment, cochleas were extracted for RT-qPCR analysis to examine the mRNA levels of various HSPs. Figure 5B shows HSP mRNA expression levels for eight members of the heat shock protein (HSP) family. Lovastatin regulated HSP expression differently depending on dose, however, both 60 mg/kg lovastatin and 40 mg/kg lovastatin significantly increased HSP60 and Hmox1 (HSP32) mRNA within the cochlea. Thus, lovastatin treatment results in an increased expression of cochlear HSPs, and this induction may be a mediator of lovastatin-induced protection against cisplatin-induced hearing loss.
Discussion
Cisplatin is a lifesaving therapy used to treat a variety of solid tumors. Many of the associated side effects of cisplatin treatment like nausea, vomiting, oral mucositis and joint pain can be effectively managed. However, ototoxicity remains a permanent impairment in affected individuals and can negatively impact the quality of life for cancer survivors (Waissbluth et al., 2017, Gentilin et al., 2019, Pearson et al., 2019). There is currently no FDA-approved clinical intervention to prevent or reverse cisplatin-induced ototoxicity. The goal of this study was to evaluate the potential for statins to reduce cisplatin ototoxicity. Our data indicate that lovastatin reduces cisplatin-induced hearing loss, and that this protection varies with both the dose of lovastatin and the sex of the subject.
Previous studies have evaluated the use of statin drugs as potential otoprotectants. Simvastatin reduces gentamicin-induced outer hair cell loss in rat cochlear cultures (Brand et al., 2011). Atorvastatin preserves OHC function in a mouse model of presbycusis (Syka et al., 2007). Both pravastatin and fluvastatin reduce noise-induced hearing loss and hair cell death (Park et al., 2012, Ritcher et al., 2018). The present study utilized a previously described and clinically relevant adult mouse model of cisplatin ototoxicity in which the treated mice exhibit a high frequency hearing loss with significant outer hair cell damage following three cycles of cisplatin administration (Fernandez et al., 2019). Our dosing paradigm results in a cumulative cisplatin dose of 36 mg/kg which approximates a human dose of ~108 mg/m2 (Nair et al., 2016), a moderate cisplatin dose in humans. Daily coadministration of lovastatin reduced cisplatin ototoxicity as measured by ABR thresholds and OHC survival.
The extent of cisplatin-induced damage to cochlear function did not differ between sexes. However, we observed a sex difference in the protective effect of lovastatin. Female mice showed protection with both 40 mg/kg and 60 mg/kg lovastatin, as measured by reduced ABR threshold shifts (Fig. 1). 60 mg/kg lovastatin provided robust protection in female mice across frequencies, with as much as 13 dB threshold improvement in the high frequencies. In contrast, male mice showed greater protection when treated with 40 mg/kg lovastatin, with ~ 22 dB threshold improvement over cisplatin alone at 22 kHz. Thus, the hearing loss caused by cisplatin is the same in male and female mice, but the protection provided by lovastatin differs between the sexes. Our study is underpowered to appropriately determine sex differences in lovastatin protection. Data reported here indicate significant protection; however, after dividing treatment groups by sex, the reduced sample sizes weakened the observed statistical power. To further examine true sex differences in statin protection, further studies are necessary using larger sample sizes, and possibly lower lovastatin doses in males. Based on the observed effects here, a priori sample size analysis indicates a minimum of 5 mice per sex are necessary to adequately determine protection with appropriate statistical power.
Women are under-represented in clinical studies of cardiovascular disease; thus, it is unclear whether there are sex differences in the therapeutic effects of statins in humans (Garcia et al., 2016, Raparelli et al, 2017). However, some studies suggest that statins may be less effective at reducing cholesterol in women than in men (Victor et al., 2014, Mombelli et al., 2015). This difference may be attributable to differences in organ size, body mass index, amount of adipose tissue, and/or plasma levels of statins between males and females. However, statins are equally effective in men and women in studies reporting prevention of cardiovascular disease (CVD) (Fulcher et al., 2015). Interestingly, Lodovici et al., (2015) examined the potential for statins to control the oxidative injury and inflammation associated with Type 2 diabetes and found women to be far more responsive than men. Statin drugs, including lovastatin, have a variety of pleiotropic effects that can reduce oxidative stress injury and inflammation (Bedi et al., 2016), two byproducts of both diabetes (Robertson and Harmon, 2006) and cisplatin treatment (Yu et al., 2018).
The bioavailability of lovastatin in the inner ear specifically is unknown. While it has been shown to cross the blood brain barrier (BBB) in mice (Hsu et al., 2006), there are currently no data indicating whether any statin crosses the blood labyrinth barrier (BLB) to the inner ear. In the current study we used 40 and 60 mg/kg/day lovastatin in male and female mice. The human equivalent dose (HED) is 3.25 and 4.88 mg/kg, respectively (Nair et al., 2016). In contrast, the typical clinical dose of lovastatin in humans is lower at 0.33–1.33 mg/kg (Jones et al., 1998). Further studies are necessary in order to optimize statin dose, and possibly statin type, to maximize the observed protective benefit in both sexes. Additional studies are also necessary to determine the longevity of the statin-mediated protection and to determine if greater protection is attainable with extended daily dosing of lovastatin (or a different statin).
The cellular and molecular mechanisms underlying lovastatin-mediated protection against cisplatin ototoxicity remain unknown and may be multifactorial. We demonstrated herein that lovastatin did not alter the uptake of cisplatin in the inner ear (Figure 5A); however, RT-qPCR data demonstrated upregulation of HSP32 (Hmox 1) and HSP60 mRNAs following lovastatin administration (Figure 5B). Induction of Hmox1 has previously been shown to inhibit cisplatin-induced hair cell death in vitro, and our current data suggest that the protective effect of lovastatin may be due at least in part to Hmox1 induction. However, the protective effect of lovastatin may also be related to its cholesterol-lowering activity via inhibition of HMG-CoA reductase, as has been suggested for statin-mediated protection against noise-induced hearing loss (Ritcher et al., 2018). Additionally, statins may promote elongation of SGN processes that form synapses with inner hair cells (Whitlon et al., 2015). In our study, the protective effect of lovastatin against cisplatin-induced hearing loss was not evident in the DPOAEs, although OHC survival was slightly higher in cisplatin mice co-treated with lovastatin relative to those not treated with lovastatin. This suggests that the protective effect of lovastatin is likely not due to improved outer hair cell function. It is possible that the improved ABR thresholds we observed are related to preservation of strial function and the endocochlear potential (EP), which is reduced in cisplatin-treated mice (Breglio et al., 2017). Our data indicate that statins are protective against cisplatin-induced hearing loss, and the mechanisms underlying this protection warrant continued future study.
Statins are relatively safe and well tolerated in humans. In general, women are underrepresented in studies evaluating the efficacy of statins in the context of cardiovascular events (Plakogiannis et al., 2016). However, multiple meta-analyses reveal that men and women show similar tolerance and benefit, as measured by LDL levels, from statins when using similar doses (Kostis et al., 2012, Cholesterol Treatment Trialists’ (CTT) Collaboration, 2015). Serious adverse events consisting of rhabdomyolysis, diabetes, and myalgia, have been associated with statin use in adults but are rare (Schachter, 2004, Ramkumar et al., 2016). Perhaps more importantly to the current study, statins are not contraindicated in patients undergoing cancer therapy. On the contrary several reports have indicated that statin use improves overall survival among patients with colorectal, breast, prostate (Zhong et al., 2015), esophageal (Deng et al., 2019), lung (Chen et al., 2019, Xia et al., 2019), and head and neck cancers (Lebo et al., 2018). As an already FDA-approved class of drugs, statins may offer a unique opportunity to safely reduce ototoxicity in patients undergoing cisplatin therapy without reducing the therapeutic efficacy of cisplatin. We are currently examining the effects of statins on hearing loss in patients undergoing cisplatin therapy (see ClinicalTrials.gov, NTC03225157 for details).
Supplementary Material
Highlights:
Cisplatin causes hearing loss in a significant proportion of treated patients
Lovastatin is a widely-used cholesterol-lowering drug that may be otoprotective
Lovastatin reduces cisplatin-induced hearing loss in a mouse model of cisplatin ototoxicity
Acknowledgements
The Authors are grateful to the animal care facility staff members of the John Edward Porter Research Neuroscience Center for their ongoing support and exceptional animal care and to Tracy Fitzgerald and the NIDCD mouse auditory testing core (project number ZIC DC000080) for expert project support. This research was funded by the Division of Intramural Research at the National Institute on Deafness and Other Communication Disorders (project number 1 ZIA DC000079).
Abbreviations:
- DPOAE
Distortion Product Otoacoustic Emissions
- ABR
Auditory Brainstem Response
- IHC
Inner Hair Cell
- OHC
Outer Hair Cell
- dB
Decibel
- SPL
Sound Pressure Level
- IP
Intraperitoneal
- ICP-MS
Inductively Coupled Plasma Mass Spectrometry
- RT-qPCR
Quantitative Reverse Transcription Polymerase Chain Reaction
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker TG, Roy S, Brandon CS, Kramarenko IK, Francis SP, Taleb M, Marshall KM, Schwendener R, Lee FS, Cunningham LL. 2015. Heat shock protein-mediated protection against cisplatin-induced hair cell death. J Assoc Res Otolaryngol, 16(1): 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi O, Dhawan V, Sharma PL, Kumar P. 2016. Pleiotropic effects of statins: new therapeutic targets in drug design. Naunyn Schmiedebergs Arch Pharmacol, 389(7): 695–712. [DOI] [PubMed] [Google Scholar]
- Blakely BW, Myers SF. 1993. Patterns of hearing loss resulting from cis-platinum therapy. Otolaryngol Head Neck Surg, 109: 385–391. [DOI] [PubMed] [Google Scholar]
- Brand Y, Setz C, Levano S, Listyo A, Chavez E, Pak K, Sung M, Radojevic V, Ryan AF, Bodmer D. 2011. Simvastatin protects auditory hair cells from gentamicin-induced toxicity and activates Akt signaling in vitro. BMC Neurosci, 12: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, Hall MD, Amable L, Cunningham LL. 2017. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nature Comm, 8: 1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo A, Sedó-Cabezón L, Juan ID, Llorens J. 2015. Cisplatin-induced ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics, 3, 268–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Huang KC, Lin WW. 2006. HMG-CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264.7 macrophages via ERK, p38 MAPK and protein kinase G pathways. Cell Signal, 18: 32–39. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ki X, Zhang R, Xia Y, Shao Z, Mei Z. 2019. Effects of statin exposure and lung cancer survival: A meta-analysis of observational studies. Pharmacol Res, 141: 357–365. [DOI] [PubMed] [Google Scholar]
- Chu Y, Sibrian-Vazquez M, Escobedo JO, Phillips AR, Dickey DT, Wang Q, Ralle M, Steyger PS, Strongin RM. 2016. Systemic delivery and biodistribution of cisplatin in vivo. Mol Pharm, 13(8): 2677–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coradini PP, Cigana L, Selistre SG, Rosito LS, Brunetto AL. 2007. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol, 29:355–360. [DOI] [PubMed] [Google Scholar]
- Deng HY, Lan X, Zheng X, Zha P, Zhou J, Wang RL, Jiang R, Qiu XM. 2019. The association between statin use and survival of esophageal cancer patients: A systematic review and meta-analysis. Medicine (Baltimore), 98(29): e16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desari S and Tchounwou PB. 2014. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol, 740: 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton Peabody Laboratories. 2018a. Tutorial for cochlear dissection. Vimeo, Uploaded by Massachusetts Eye and Ear Infirmary. www.masseyeandear.org. Accessed 25 May 2019.
- Eaton Peabody Laboratories. 2018b. ImageJ Plugin for Cochlear Frequency Mapping in Whole Mounts. www.masseyeandear.org. Accessed 25 May 2019.
- Fernandez KA, Wafa T, Fitzgerald TS, Cunningham LL. 2019. An optimized, clinically relevant mouse model of cisplatin-induced ototoxicity. Hear Res, 275: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher J, O’Connell, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi M, Baigent C, Keech A. Cholesterol Treatment Trialists’ (CTT) Collaboration. 2015. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomized trials. Lancet, 385: 1397–1405. [DOI] [PubMed] [Google Scholar]
- Francis SP, Kramarenko II, Brandon CS, Lee FS, Baker TG, Cunningham LL. 2011. Celastrol inhibits aminoglycoside-induced ototoxicity via heat shock protein 32. Cell Death Disease, 2: e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Mulvagh SL, Merz CNB, Buring JE, Manson JE. 2016. Cardiovascular disease in women: clinical perspectives. Circ Res, 118: 1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilin E, Simoni E, Candito M, Cazzador D, Astolfi L. 2019. Cisplatin-induced ototoxicity: Updates on molecular targets. Trends Mol Med, 28: doi: 10.1016/j.molmed.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Helm CW, States JC. 2009. Enhancing the efficacy of cisplatin in ovarian cancer treatment – could arsenic have a role. J Ovarian Res, 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnloser JH, Schierl R, Hasford B, Emmerich B. 1995. Cisplatin based chemotherapy in testicular cancer patients: long term platinum excretion and clinical effects. Eur J Med Res, 1: 509–514. [PubMed] [Google Scholar]
- Hosick PA, Weeks MF, Hankins MW, Moore KH, Stec DE. 2017. Sex-dependent effects of HO-1 deletion from adipocytes in mice. Int J Mol Sci, 18: 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Muchova L, Morioka I, Wong RJ, Schröder H, Stevenson DK. 2006. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem Biophys Res Comm, 343: 738–744. [DOI] [PubMed] [Google Scholar]
- Jones P, Kafonek S, Laurora I, Hunninghake D. 1998. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and Fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol, 81(5): 582–7. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, Park R, So HS. 2010. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci, 30(11): 3933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling J. 2003. FDA contemplates collection of pharmacogenomic data. Nat Biotechnol, 21: 590. [DOI] [PubMed] [Google Scholar]
- Knight KR, Kraemer DF, Neuwelt EA. 2005. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol,23: 8588–8596. [DOI] [PubMed] [Google Scholar]
- Köppen C, Reifschneider O, Castanheira I, Sperling M, Karst U, Ciarimnoli G. 2015. Quantitative imaging of platinum based on laser ablation-inductively coupled plasma-mass spectrometry to investigate toxic side effects of cisplatin. Metallomics, 00: 1–3. [DOI] [PubMed] [Google Scholar]
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. 2012. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol, 59(6): 572–82. [DOI] [PubMed] [Google Scholar]
- Lebo NL, Griffiths R, Hall S, Dimitroulakos J, Johnson-Obaseki S. 2018. The effect of statin use on oncologic outcomes in head and neck squamous cell carcinoma. Head Neck,40(8): 1697–1706. [DOI] [PubMed] [Google Scholar]
- Lee TS, Chang CC, Zhu Y, Shyy JY. 2004. Simvastatin induces heme oxygenase-1: A novel mechanism of vessel protection. Circulation, 110(10): 1296–1302. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, DuBois SG, Fligor B, Li X, Goorin A, Grier HE. 2009. Ototoxicity in children treated for osteosarcoma. Pediatr Blood Cancer, 52: 387–391. [DOI] [PubMed] [Google Scholar]
- Maines MD, Trakshel GM. 1992. Differential regulation of heme oxygenase isozymes by Sn- and Zn-protoporphyrins: possible relevance to suppression of hyperbilirubinemia, Biochim, Biophys Acta, 1131(2): 166–174. [DOI] [PubMed] [Google Scholar]
- Martindale JL and Holbrook NJ. 2002. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol, 192: 1–15. [DOI] [PubMed] [Google Scholar]
- May LA, Kramarenko II, Brandon CS, Voelkei-Johnson C, Roy S, Truong K, Francis SP, Monzack EL, Lee FS, Cunningham LL. 2013. Inner ear supporting cells protect hair cells by secreting HSP70. J Clin Invest, 123: 3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. 2016. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin, 66: 271–89. [DOI] [PubMed] [Google Scholar]
- Mombelli G, Bosisio R, Calabresi L, Magni P, Pavanello C, Pazzucconi F, Sirtori CR. 2015. Gender-related lipid and/or lipoprotein responses to statins in subjects in primary and secondary prevention. J Clin Lipidol., 9: 226–233. [DOI] [PubMed] [Google Scholar]
- Müller M, von Hünerbein K, Hoidis S, Smolders JH. 2005. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res, 202:63–73. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S. 2016. A simple practice guide for dose conversion between animals and humans. J Basic Clin Pharm,7: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (NIH). 2018. Fact sheet – cancer. http://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=75. Accessed 30 August 2019.
- Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A. 2019. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Science Translational Medicine, 11(482): eaao0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson SE, Taylor J, Patel P, Baguley DM. 2019. Cancer survivors treated with platinum-based chemotherapy affected by ototoxicity and the impact on quality of life: a narrative synthesis systematic review. Int J Audiol, September 23: 1–11. [DOI] [PubMed] [Google Scholar]
- Ramkumar S, Raqhunath A, Raqhunath S. 2016. Statin therapy: Review of safety and potential side effects. Acta Cardiol Sin, 32(6): 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raparelli V, Pannitteri G, Todisco T, Toriello F, Napoleone L, Manfredini R, Basili S. 2017. Treatment and response to statins: Gender-related differences. Curr Med Chem, 24(24): 2628–2638. [DOI] [PubMed] [Google Scholar]
- Richter CP, Young H., Richter SV, Smith-Bronstein V, Stock SR, Xia X, Soriano C, Whitlon DS. 2018. Fluvastatin protects cochlea from damage by high-level noise. Sci Rep, 8: 3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RP, Harmon JS. 2006. Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med, 41: 177–184. [DOI] [PubMed] [Google Scholar]
- Ross CJ, Katzov-Eckert H, Dubé MP, Brooks B, Rassekh SR, Barhdadi A, Feroz-Zada Y, Visscher H, Brown AM, Rieder MJ, Rogers PC, Phillips MS, Carleton BC, Hayden MR, CPNDS Consortium. 2009. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet 41(12): 1345–9. [DOI] [PubMed] [Google Scholar]
- Schachter M. 2004. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundamental & Clinical Pharmacology, 19: 117–125. [DOI] [PubMed] [Google Scholar]
- Schmitt NC, Page BR. 2018. Chemoradiation-induced hearing loss remains a major concern for head and neck cancer. Int J Audiol, 57: S48–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner R, Pearson AD, Amineddine HA 1990. Ototoxicity of cisplatinum in children and adolescents. Br J Cancer, 61: 927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Ouda L, Nachtigal P, Solichová D, Semecky V. 2007. Atorvastatin slows dow the deterioration of inner ear function with age in mice. Neurosci Lett, 411(2): 112–6. [DOI] [PubMed] [Google Scholar]
- Taleb M, Brandon CS, Lee FS, Lomax MI, Dillman WH, Cunningham LL. 2008. Hsp70 inhibits aminoglycoside-induced hair cell death and is necessary for the protective effect of heat shock. J Assoc Res Otolaryngol, 9:2770289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb M, Brandon CS, Lee FS, Harris KC, Dillman WH, Cunningham LL. 2009. Hsp inhibits aminoglycoside-induced hearing loss and cochlear hair cell death. Cell Stress Chaperones, 14(4): 427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen EAR, Bosma SCJ, Zuur CL, Spijker R, van der Baan S, Dreschler WA, de Boer JP, Balm AJM, Rasch CRN. 2015. Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy: A systemic review of the literature. Head Neck, 37(2): 281–292. [DOI] [PubMed] [Google Scholar]
- Thomas JP, Lauterman J, Leidert B, Seiler F, Thomale J. 2006. High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity. Mol Pharmacol, 70: 23–29. [DOI] [PubMed] [Google Scholar]
- Ullman-Culleré MH, Foltz CJ. 1999. Body conditioning scoring: a rapid and accurate method for assessing health status of mice. Lab Anim Sci, 49: 319–323. [PubMed] [Google Scholar]
- van Rujiven, de Groot JC, Klis SF, Smorrenburg GF. 2005. The cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hear Res, 205: 241–248. [DOI] [PubMed] [Google Scholar]
- Victor BM, Teal V, Ahedor L, Karalis DG. 2014. Gender differences in achieving optimal lipid goals in patients with coronary artery disease. Am J Cardiol, 113: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Waissbluth S, Peleva E, Daniel SJ. 2017. Platinum-induced ototoxicity: a review of prevailing ototoxicity criteria. Eur Arch Otorhinolaryngol, 274: 1187–1196. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Grover M, Dunne SF, Ritcher S, Luan CH, Ritcher CP. 2015. Novel high content screen detects compounds that promote neurite regeneration from cochlear spiral ganglion neurons. Sci Rep, 5:15960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia DK, Hu ZG, Tian YF, Zeng FJ. 2019. Statin use and prognosis of lung cancer: a systematic review and meta-analysis of observational studies and randomized controlled trials. Drug Des Devel Ther, 13: 405–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Chen Y, Dubrulle J, Stossi F, Putluri V, Sreekumar A, Puturi N, Baluya D, Lai SY, Sandulache VA. 2018. Cisplatin generates oxidative stress which is accompanied by rapid shifts in central carbon metabolism. Sci Rep, 8(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. 2015. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Drug Des Devel Ther, 13: 405–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


