Abstract
Novel psychoactive substances (NPS), especially those newly created, are largely an unknown quantity, particularly in terms of their potential serious adverse effects. This means that policy‐makers and clinicians are under‐informed about appropriate responses. Collation of detailed information on deaths related to NPS use can help in providing knowledge and understanding these aspects of the NPS phenomenon. The purpose of this review is to outline the role(s) which such evidence‐based data can play in this respect. UK NPS‐related cases demonstrate differences in definitions used by the General Mortality Registers, and differences between countries, not only in terms of the type of NPS implicated in deaths, but the number and extent of such deaths over time. NPS deaths are continuing to increase numerically and as a proportion of all drug‐poisoning deaths. In order to better understand how specific molecules contribute to and/or cause death, detailed information collected by Special Mortality Registers can provide examples of substances' modes of action, adverse effects, symptomatology, treatment interventions, mechanisms of death, etc. This information can provide clinicians and policy‐makers with objective information on the serious harms from such emerging molecules. Such evidence‐based advice informs public health interventions, service provision and policy decisions on regulation and control of NPS. However, without reliable, accurate and complete information that is correctly collated, scientifically analysed and disseminated in a timely manner, an understanding of the phenomenon of what deaths can be ascribed to NPS, their characteristics and nature will remain unachieved, and thus limit what can be done to reduce them.
Keywords: clinicians, deaths, information needs, novel psychoactive substances, policy‐makers
1. INTRODUCTION
Deaths are the ultimate indicator of the serious consequences that can arise out of administering drugs, whether to oneself or another, intentionally or accidentally.1 This fact is true no matter whether the drug concerned is controlled or regulated under the United Nations' Conventions, national legislation (such the Misuse of Drugs Act 1971 and Psychoactive Substances Act 2016 in the United Kingdom [UK]), is a prescription‐only medication (POM), is an over‐the‐counter (OTC) product, is a natural product derived from flora and fauna, or is something totally synthetic and newly created/discovered.
Careful detailed examination of information on mortality associated with drug use facilitates systematic analyses that can reveal the characteristics of individuals dying as a consequence of drug toxicity. Gaining an understanding of such characteristics, the circumstances and characteristics of the deaths themselves, is fundamental and intrinsic for generating a robust evidence base on harms related to specific drugs, as well as providing clinical and policy intervention opportunities.
In many instances, a lot is known about substances that have been used for therapeutic and/or recreational purposes over decades or even centuries, including their properties in respect of action on neurotransmitters, potential for addiction/dependence, chemistry, metabolism, pharmacodynamics, pharmacokinetics, pharmacology, etc. Furthermore, POMs and OTCs will have undergone pre‐clinical (animal) and clinical (human) trials before being licensed for therapeutic use. Some drugs may have demonstrated undesirable or adverse effects during these trials and subsequently not been released; some adverse consequences may have come to light following licensing and the drugs are then withdrawn from the market. Such drugs may later be ‘rediscovered’ and promoted for recreational purposes, but their full mode of action, effects and toxicity may well not be fully understood, especially at supra‐therapeutic dosages.
Understanding the nature and properties of more traditional psychoactive drugs and the characteristics of how they contribute to and/or cause death has provided policy‐makers and clinicians with the necessary knowledge and tools to try and reduce such events and to provide suitable interventions and treatments. One example of such research feeding into practice is that of Farrell and Marsden,2 who examined drug‐related deaths (DRDs) of newly released prisoners and found that such deaths among males often involved heroin, whilst those of females featured benzodiazepines and cocaine. Amongst other suggestions, they recommended prevention measures such as opioid maintenance therapy being introduced in prisons. A subsequent study found that there was a reduction of 75% in all‐cause mortality and an 85% fall in drug‐related poisoning deaths in the first month post‐release where prison‐based opioid substitution therapy had been introduced.3
Novel psychoactive substances (NPS) present policy‐makers, clinicians and other stake‐holders with several challenges. First of all, and perhaps the most pressing, is the fact that, for the most part, they are likely to be brand new substances about which little or nothing is known about either their short‐ or long‐term effects. Unless, they are existing drugs, it is unlikely that newly created NPS will have undergone pre‐clinical trials. A pre‐market approval regulatory scheme was set up in New Zealand in July 2013 with a view to producing clinical trial data capable of demonstrating that NPS products posing a ‘low risk’ of harm would be suitable for legal manufacture and sale.4, 5, 6 However, this does not appear to have been viewed as a success by regulators, retailers or consumers. Although some researchers may have tried out the ‘designer drugs’ they created on themselves and even written down their experiences as objectively as they could,7 such anecdotal reports are no substitute for properly conducted pre‐clinical and clinical trials which can inform their subsequent use.
A further challenge is the fact that NPS are subject to rapid diversification and continual appearance in drug markets. The number of newly created NPS has increased rapidly in the last decade or so, largely as a result of legislation being introduced to reduce the production, importation, sale and availability of such molecules. For example, in the European Union the number of NPS identified and reported for the first time to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) increased from 7 in 2006 to 101 in 2014.8 Although the rate of notification has fallen (to 55 in 2018), the number of molecules in certain groups continues to grow, especially synthetic cannabinoids and synthetic cathinones. Of concern in recent times has been the increasing appearance of highly potent new synthetic opioids, chiefly fentanyl analogues.
The consumption of NPS (4.7% last‐year use) is much lower than that of traditional recreational drugs such as cocaine (10.7%), ecstasy (11.3%) and cannabis (30.7%) in England and Wales.9 As prevalence is low, insights into the use and harms caused by NPS are much more difficult to measure. Most population‐based, especially household, surveys may only ask about NPS as a whole or only one specific molecule (typically mephedrone); they are also unlikely to capture specific sub‐populations of NPS users, such as prisoners, students, the homeless and ‘roofless’ and ‘psychonauts’.1 Even targeted surveys, such as the Global Drug Survey,10 are only likely to capture information on the more commonly used NPS, rather than newly emerging ones.
These gaps in knowledge and understanding can be filled by using mortality data; they document the most serious adverse consequences arising from NPS use, including those less commonly consumed. The objectives of this review are to outline how information derived from investigations into deaths can help clinicians and policy‐makers gain a better understanding of NPS and the role(s) which such evidence‐based data can play in dealing with the phenomenon. The current situation regarding information on UK NPS‐related deaths, what they can tell us, their limitations and future requirements, is examined. Likely developments in the evolution of NPS deaths are presented. Above all, this study aims to demonstrate that without reliable, accurate and complete information that is correctly collated, scientifically analysed and disseminated in a timely manner, an understanding of the phenomenon of what deaths can be ascribed to NPS and their characteristics and nature will remain unachieved.
2. SOURCES OF PUBLISHED INFORMATION ON UK DRUG‐RELATED DEATHS
There are two main types of source for information on ‘acute’ DRDs, that is, fatalities directly related to drug consumption, in the UK: (a) three General Mortality Registers (GMRs), the General Register Offices for England & Wales (whose data is analysed by the Office for National Statistics), Scotland (National Records for Scotland) and Northern Ireland (the data from the latter is analysed by the Northern Ireland Statistics and Research Agency, NISRA), which all use ICD 10,11 and (b) two Special Mortality Registers (SMRs), the National Programme on Substance Abuse Deaths (NPSAD) based at St George's, University of London, since 1997 and, since the beginning of 2009, a national database hosted by the Information Statistics Division (ISD) Scotland.
There are substantive differences between these two types of sources. The GMRs contain information, derived from medical death certificates, on the cause of death and intentionality, but no information on toxicology, autopsy and little information from coroner's findings. In England, Wales and Scotland some supplementary information is provided.12, 13 The details of substances recorded by the GMRs are those recorded on the death certificate. No detailed information is passed to them on toxicology, e.g., levels of drugs and/or alcohol found in body tissues, blood or urine, neither are post‐mortem reports. However, in Scotland the names of other substances found at post mortem are listed, together with those involved in the death.12
By contrast, the SMRs have more detailed information, especially about toxicology and pathology. NPSAD receives information on a voluntary basis from coroners across the UK, including information on prescribed medication, socio‐demographics, history of drug use and injecting, pathology and toxicology, as well as details of circumstances surrounding the death.14 Data collated for England and Wales, however, are based on information that is voluntarily provided by coroners across the country and may not be representative of all cases. The National Drug‐Related Deaths Database in Scotland contains personal details gathered about the drug user including information on: their drug taking history, where they were living and with whom (including children), whether they were known to services or were on waiting lists, involvement with the criminal justice system, what drugs were found at the scene and in the their toxicology, whether they were taking methadone or other drugs, and whether the drugs were prescribed to them or not. The database is cross‐referenced with other ISD data sources such as those for hospital discharges, psychiatric discharges and the Scottish Drug Misuse Database.15
3. LIMITATIONS OF UK MORTALITY DATA ON NPS
Against the general picture of the national data sources noted above, there are several issues which need to be borne in mind when using information provided by or derived from them.
Historically, it was difficult to identify ‘unknown’ substances, especially NPS, as it was difficult and expensive to obtain reference samples against which to compare them. A decade ago, reference samples had to be made to order, using techniques for synthesis that had to be specially developed, were time‐consuming to design and produced only small quantities, which, in turn, made them expensive. This meant that absolute confirmation of a molecule's identity could take time, with repercussions for timely investigations and treatment advice.
The identification of NPS metabolites and/or the parent molecule can be difficult in biofluids due to the low concentrations used in more potent substances (eg, fentanyls and NBOMes), as well as the scant knowledge that exists about them.16 For example, carfentanil is estimated to be more than 10 000 times more potent than morphine, and the extremely low concentrations of some synthetic fentanyls are not always revealed by standard toxicological screening methods.17
Even where reference samples are available, other issues exist, including the technology available. As up‐to‐date technical equipment is required to deal with post‐mortem samples that may contain ‘unknown’ NPS, screening for them is usually limited to specialised laboratories. Whilst liquid chromatography‐mass spectrometry (LC‐MS/MS) is suitable for the detection of fentanyl, the use of thermal desorption direct analysis in real‐time mass spectrometry (TD‐DART‐MS) and ion mobility spectrometry (IMS) are required for the rapid and sensitive (nanogram to picogram) detection of its analogues such as acetyl fentanyl, carfentanil, furanyl fentanyl and isobutyryl fentanyl.18 Special facilities are also needed to use computational software tools (in vitro) in approaches for predicting molecular structures in forensic cases of suspected toxicity but where targeted analysis has not identified a drug.19 There is variability in the availability of such resources across UK forensic providers.
In the UK the decision on whether or not to conduct an autopsy in a suspected DRD is that of the relevant Coroner (Northern Ireland, England and Wales) or Procurator Fiscal (Scotland). It is unlikely for an autopsy to be needed if a death occurs in hospital and the cause of death is known. For similar reasons, it is also unlikely that an inquest or fatal accident inquiry would take place.
The proportion of all deaths reported to coroners in England and Wales in which an autopsy was conducted has fallen from 61% in 1995 to 39% in 2018, mirroring the general picture for cases where no inquest was held (down from 56% to 34% over the same period). However, where inquests were held, the proportion having a post‐mortem examination fell from 98% to 62%. Between 2011 and 2018, the proportion of post‐mortems with toxicology rose from 13% to 21%.20 Unfortunately, the extent and use of post‐mortem examinations and toxicology investigations in DRDs is unknown at the national level. However, it is likely that the levels of both post‐mortem and toxicological investigations are higher in deaths suspected to be drug‐related, especially where the deceased is a known drug user and/or the circumstances of death suggest this possibility. That said, it is likely that some deaths may remain unidentified as being related to drug use, in part due to possible NPS use going unrecognised.
In the UK, as in most parts of the world, there are no national standards or list of what substances should be screened for by forensic providers, whether medications or illicit drugs, let alone “unknown” substances. Decisions about such investigations are likely to be made on a case‐by‐case basis unless a cluster of deaths has occurred in a locality or involving individuals with common interests or backgrounds. As with DRDs in general, the circumstantial, eye‐witness and forensic information available to investigators will suggest whether toxicology is needed, and if so what substances should be searched for and identified. Post‐mortem investigations, including autopsy and histology, will help to inform forensic providers what possible culprits may be lurking in the body tissues. Toxicological screening for the “usual suspects” may identify common/popular substances that are known to cause/contribute to death, especially if they produce well‐recognised symptomatology to which a cause of death can be attributed. This may result in no further screening taking place, and thus any potential NPS and their role(s) in death remain under‐investigated and unidentified. Costs to coroners and other investigators will also play a part in such decisions, as will the resources (reference samples, reference libraries of spectra and other analytical data, and specialist equipment) available to the forensic providers employed, limiting their ability to appreciate the potential role of novel NPS. As there are differences in practices and toxicological investigation sensitivity levels between laboratories and between countries, there are issues in comparing data.21 Synthetic cathinones (82%), phenethylamines (71%), followed by synthetic cannabinoids and piperazines are the most commonly screened for NPS in Europe.21 To assist in providing some guidance on other substances, a list of NPS found in French DRD toxicological samples was issued.22 For the reasons outlined above, the presence of and possible contribution to deaths by NPS are underestimated.21 However, the extent of this under‐estimate remains unknown.
Identifying and understanding the possible contribution to or role of a drug in a fatality requires information on a range of aspects as outlined above. These processes of attribution are made infinitely more difficult, if not almost impossible, in the case of most NPS due to a lack of information on what constitute recreational, therapeutic, toxic and lethal doses and post‐mortem toxicological levels. This is so even when a sole NPS molecule is found, let alone when more than one NPS is detected and/or other drugs are present, which is often the case.23 The interaction of new molecules with other substances is largely unknown and can only be guessed at or inferred based on similar known chemical structures, i.e. by analogy. Furthermore, the way in which a specific NPS may affect the human body is unlikely to be known in advance due to the lack of pre‐clinical trials. Indeed, it is such deaths that themselves assist in helping to create an evidence base.
The accurate recording of substances implicated and/or contributing to death on medical death certificates has long been a concern for vital statisticians and substance epidemiologists alike.24, 25 For example, only some of the substances involved may be listed, or just a general description may be given (“prescribed medication”, “painkillers”, “opioid‐type”). Furthermore, only “drug overdose” or “multiple drug toxicity” is stated in about 12% of drug poisoning.26 In the past, this worry was in relation to traditional drugs of abuse, since if such details are omitted the number of DRDs attributed to specific molecules will be an undercount.27 Today, such issues also affect the identification and recording of NPS in the cause of death. This is likely to be the case where the pathologist and/or coroner/procurator fiscal may only have a limited understanding of NPS in general, and no awareness of the importance of distinguishing between different molecules within the same chemical family, eg, cathinones. In the case of NPS this means that death certificates are of limited epidemiological use.
Published statistics on drug‐related poisoning deaths within the UK are generated using extraction processes from the general deaths database based on International Classification of Disease (ICD) codes and manual extraction of chemical names, etc. However, in 1993 the ONS developed a separate drug poisonings database, and this was followed by a similar approach in Scotland in 1994 and Northern Ireland in 1997. However, there are issues with the use of ICD‐10,11 introduced into use in the UK in 1999‐2000, in respect of accurately identifying and classifying both recreational drug hospital admissions28, 29 and drug deaths,30 including those involving NPS.29 As we will see below, the next version of the ICD contains some provision for NPS, but the process for updating this classification is very slow and does not respond in a timely way to accommodate newly emerging substances.
The reporting of deaths and their subsequent collation, analysis and publication of statistics on DRDs present with several limitations. An inquest into a death, including a DRD, typically takes place about 6 months after the fatal event, and it then takes up to another 12 months before that death appears in the national statistics published by the GMR. This means that although the statistics are comprehensive and useful in identifying long‐term trends, they are not timely from an intelligence or early‐warning perspective. By the time stake‐holders are aware of these changes, several other NPS with different properties and more serious consequences may have emerged and the ones reported on ceased to be of interest to consumers.
Moreover, the effects of legislation on NPS definitions and lack of continuity in definition by GMRs causes issues in monitoring not only sole molecules but also classes of NPS.31 This is because the status of a specific molecule may change from being regarded as a psychoactive substance (including medications exempted from the provisions of the Psychoactive Substances Act 2016) and hence an NPS to subsequently being controlled under the Misuse of Drugs Act 1971, which means it is no longer within the NPS category. Consequently, the pool of molecules regarded as forming the NPS group is changing over time.
One of the key issues about looking at NPS‐related deaths is the fact that there are differences between UK GMRs in how they define NPS.1 For detailed information look at the technical information accompanying their most recent published statistics.32, 33, 34 A summary is provided in Table 1. It should be noted that in the case of the ONS listings, the list of substances included is incomplete, eg, deaths have occurred where plants such as Iboga (Ibogaine) and Kratom (Mitragynine) have been mentioned in the cause of death.
Table 1.
Definitions of novel psychoactive substances used by General Mortality Registers in the UK
| Agency | Definition | Substances included |
|---|---|---|
| Office for National Statistics (ONS) |
List of drugs included in the ONS new psychoactive substances grouping. The groups given in the published table are cathinones (of which mephedrone is given separately), GHB (which is understood to include GBL), benzodiazepine analogues, methiopropamine, alpha‐methyltryptamine, benzofurans, novel amphetamines, novel opiates, piperazine derivatives, NBOMEs, synthetic cannabinoids, other NPS The specific substances included in each category are not stated |
The specific substances listed in 2018 (in numeric‐alphabetical order) are 1‐(benzofuran‐5‐yl)‐N‐methylpropan‐2‐amine, 1‐(benzofuran‐5‐yl)‐propan‐2‐amine, 1‐(benzofuran‐6‐yl)‐propan‐2‐amine, 2‐(1H‐indol‐5‐yl)‐1‐methylethylamine, 25B‐NBOMe, 25C‐NBOMe, 25I‐NBOMe, 2‐aminoindane, 2‐diphenylmethylpyrrolidine, 3‐FPM, 3‐methoxyphencyclidine, 4,4′‐DMAR, 4‐fluoroephedrine, 4‐fluoromethcathinone, 4‐methoxymethcathinone, 4‐methylamphetamine, 4‐methylethcathinone, 4‐methylpentedrone, 5‐EAPB, 5F‐ADB, 5F‐AKB‐48, 5F‐PB‐22, AB‐CHMINACA, AB‐FUBINACA, acetylfentanyl, AH‐7921, alpha‐methyltryptamine, alpha‐PVP, APB, APDB, benzylfentanyl, butylone, BZP, cathine, cathinone, clephedrone, cyclopropylfentanyl, desoxypipradrol, diclazepam, diphenidine, EAPB, ethylphenidate, etizolam, flubromazepam, flubromazolam, fluoromethamphetamine, fluoromethcathinone, furanylfentanyl, GHB, khat, MDDA, MDMB‐CHMICA, MDPHP, mephedrone, methiopropamine, methoxetamine, methoxphenidine, methoxyacetylfentanyl, methylenedioxypyrovalerone, methylethcathinone, methylone, mexedrone, N‐methyl‐3‐phenyl‐norbornan‐2‐amine, ocfentanil, phenazepam, pyrazolam, synthetic cannabinoid, TFMPP, U‐47700 |
| Northern Ireland Statistics & Research Agency (NISRA) | NISRA uses the same approach as used by ONS | The specific substances listed in 2017 (in numeric‐alphabetical order) are 1‐(benzofuran‐5‐yl)‐N‐methylpropan‐2‐amine, 1‐(benzofuran‐5‐yl)‐propan‐2‐amine, 1‐(benzofuran‐6‐yl)‐propan‐2‐amine, 2‐(1H‐indol‐5‐yl)‐1‐methylethylamine, 25B‐NBOMe, 25C‐NBOMe, 25I‐NBOMe, 2‐diphenylmethylpyrrolidine, 4,4′‐DMAR, 4‐fluoroephedrine, 4‐fluoromethcathinone, 4‐methoxymethcathinone, 4‐methylamphetamine, 4‐methylethcathinone, 5‐EAPB, 5F‐AKB‐48, 5F‐PB‐22, AB‐CHMINACA, acetylfentanyl, AH‐7921, alpha‐methyltryptamine, APB, APDB, butylone, BZP, cathinone, desoxypipradrol, diclazepam, diphenidine, EAPB, ethylphenidate, etizolam, flubromazepam, flubromazolam, fluoromethamphetamine, fluoromethcathinone, GHB, khat, MDDA, MDMB‐CHMICA, mephedrone, methiopropamine, methoxetamine, methoxphenidine, methylenedioxypyrovalerone, methylethcathinone, methylone, N‐methyl‐3‐phenyl‐norbornan‐2‐amine, phenazepam, pyrazolam, synthetic cannabinoid, TFMPP. |
| National Records of Scotland (NRS) |
“The term ‘New Psychoactive Substances’ (NPS) is meant to cover the kinds of substances that people have, in recent years, begun to use for intoxicating purposes. In general, when an NPS first became available it would not have been a controlled substance under the misuse of Drugs Act 1971. Some NPS may still not be controlled under that Act; if so, they will be covered by the Psychoactive Substances Act, which came into force on 26 May 2016. The definition of NPS therefore includes substances which some people have described as ‘legal highs’ (by which is meant substances which were legally available at the time of the death, whether or not they have since become controlled under the Misuse of Drugs Act or become subject to the Psychoactive Substances Act).” The substances are then categorised into three groups as detailed in the adjacent column |
|
Abbreviations: GBL, gamma‐Butyrolactone; GHB, gamma‐Hydroxybutyric acid; MDPHP, 3',4'‐Methylenedioxy‐α‐pyrrolidinohexiophenone; MDDA, 3,4‐methylenedioxy‐N,N‐dimethylamphetamine; NBOME, N‐methoxybenzyl.
Note. The molecules listed are only those which have been implicated in the cause of death of cases registered within that agency's jurisdiction.
4. DEATHS RELATED TO NPS IN THE UK
Table 2 shows that in respect of fatalities registered by the UK GMRs there was a gradual increase in the number of NPS‐related cases from 2007 to 2009, followed by a faster rate of increase. However, the rate of increase was not uniform across the three GMRs. For example, in Northern Ireland deaths registered quadrupled between 2013 and 2014, followed by another relatively high number in 2015; much of this increase was due to a particular stimulant and entactogen (4,4′‐dimethylaminorex or 4,4′‐DMAR), which became controlled as a Class A drug on 11 March 2015. In Scotland, a tripling of numbers occurred between 2015 and 2016, largely due to an explosion of deaths involving ‘designer’ benzodiazepines. This trend continued into 2017 and 2018 with a further increase of 162%,12, 32 which was particularly due to etizolam and alprazolam. In England and Wales the growth in NPS death registrations was less pronounced, but there was a pronounced drop in 2017, likely due to several existing NPS becoming controlled that year and thus dropping out of the NPS definition used by the ONS. However, in 2018 the total returned to its 2016 level, with synthetic cannabinoids, GHB and synthetic cathinones accounting for most deaths.33 These differences highlight the role of different drugs in the various parts of the UK, echoing the picture in the wider range of deaths related to drug poisonings.
Table 2.
Number of deaths related to drug poisonings and NPS, and percentage of NPS to drug cases, registered by country and year, UK, 2007‐2018
| Country | Year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2007‐2018 |
| NPS1 | |||||||||||||
| England and Wales | 9 | 25 | 26 | 22 | 31 | 55 | 63 | 82 | 114 | 123 | 61 | 125 | 736 |
| Northern Ireland | 0 | 0 | 4 | 2 | 2 | 2 | 4 | 19 | 16 | 7 | 12 | NA | 68 |
| Scotland | 0 | 0 | 4 | 11 | 47 | 47 | 113 | 114 | 112 | 346 | 363 | 5882 | 1745 |
| UK | 9 | 25 | 34 | 35 | 80 | 104 | 180 | 215 | 242 | 476 | 436 | 713 | 2549 |
| Drug poisonings3 | |||||||||||||
| England and Wales | 2640 | 2928 | 2878 | 2747 | 2652 | 2597 | 2955 | 3346 | 3674 | 3744 | 3756 | 4359 | 38 276 |
| Northern Ireland | 86 | 89 | 84 | 92 | 102 | 110 | 115 | 110 | 144 | 127 | 136 | NA | 1195 |
| Scotland | 630 | 737 | 716 | 692 | 749 | 734 | 685 | 743 | 813 | 997 | 1,045 | 1313 | 9854 |
| UK | 3356 | 3754 | 3678 | 3531 | 3503 | 3441 | 3755 | 4199 | 4631 | 4868 | 4937 | 5672 | 49 325 |
| Percentage of NPS: drug poisonings | |||||||||||||
| England and Wales | 0.34 | 0.85 | 0.90 | 0.80 | 1.17 | 2.12 | 2.13 | 2.45 | 3.10 | 3.29 | 1.62 | 2.87 | 1.92 |
| Northern Ireland | 0.00 | 0.00 | 4.76 | 2.17 | 1.96 | 1.82 | 3.48 | 17.27 | 11.11 | 5.51 | 8.82 | NA | 5.69 |
| Scotland | 0.00 | 0.00 | 0.56 | 1.59 | 6.28 | 6.40 | 16.50 | 15.34 | 13.78 | 34.70 | 34.74 | 44.78 | 17.71 |
| UK | 0.27 | 0.67 | 0.92 | 0.99 | 2.28 | 3.02 | 4.79 | 5.12 | 5.23 | 9.78 | 8.83 | 12.57 | 5.17 |
Note 1. There are differences in NPS definitions between different parts of the UK.
Note 2. The majority of these NPS deaths were due to benzodiazepines, namely etizolam (98%, 577/588).
Note 3. Using the ONS “wide” definition of drug‐poisoning deaths: ICD‐10 codes F11‐F16, F18‐F19, X40‐X44, X60‐X64, X85, Y11‐Y14.
Abbreviations: NA, not applicable.
Comparing countries within the UK in respect of the proportion of all drug‐related poisoning deaths, overall in the period 2007‐2017 Scotland had the highest at 13.55%, compared to 5.69% for Northern Ireland and just 1.80% for England and Wales (Table 2). Of note, is that NPS‐related deaths accounted for one‐third of all Scottish drug‐poisoning deaths in both 2016 and 2017, rising to 45% in 2018; there is a lower proportion in Northern Ireland in recent years, but the still elevated rate presents a challenge for policy‐makers. The increased proportions in 2014 and 2015 are likely due to cases involving 4,4′‐DMAR; the drop in following years is likely due to legislative changes (noted above).
The difference between countries is further emphasised by examining the rate of NPS‐related deaths registered per 1 000 000 population (Figure 1). For the period 2007‐2018, Scotland had a mean rate of 27.35 compared to 3.39 for Northern Ireland (2007‐2017) and only 1.08 for England and Wales. There is a higher rate of DRDs generally in Scotland and Northern Ireland compared to England and Wales. For example, for 2017 death registrations, the rate for drug‐poisoning deaths in Scotland is at least double that in any other part of the UK, and three times that for England (Table 3), but the rate in Scotland increased again in 2018.32 These patterns are reflected in NPS‐related deaths. However, it should be noted that it is NPS deaths which have driven this increasingly growing difference in recent years.
Figure 1.
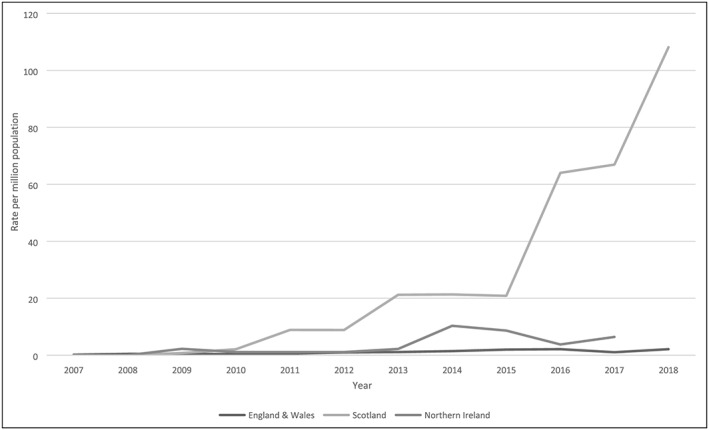
Rate of NPS poisoning deaths per 1 000 000 population, by country and year, UK, 2007‐2018
Table 3.
Drug‐related death mortality rates per 1 000 000 population, by country, UK, 2017 and 2018
| Country | Drug poisoning deaths | |||
|---|---|---|---|---|
| 2017 | 2018 | |||
| Number | Rate per million population | Number | Rate per million population | |
| England | 3482 | 62.6 | 3,983 | 71.15 |
| Wales | 260 | 83.1 | 327 | 104.19 |
| Northern Ireland | 136 | 72.6 | NA | NA |
| Scotland | 1045 | 192.6 | 1,313 | 241.44 |
| UK | 4923 | 74.5 | NA | NA |
So far as the authors are aware, there are no published studies looking specifically either at the homogeneity or at the heterogeneity of NPS‐related deaths between different countries, or between regions within individual countries, although reference has been made by us to there being differences.1
Let us look briefly at the number of deaths by selected NPS drug groups registered for the period 2013‐2018; published information for Scotland on a case‐by‐case basis is only available from 2015 onwards, data for Northern Ireland on this basis are unpublished and only available to the lead author for the period up to 2016. Taking this 6‐year period as a whole, the vast majority of deaths are related to benzodiazepine analogues (‘designer benzos’), followed by synthetic cathinones, GHB/GBL, synthetic cannabinoids and “other” (Table 4). However, this general statement hides some interesting differences over time and between countries. For example, most of the benzodiazepine analogues feature in Scotland in 2016‐2018, whereas in England and Wales synthetic cathinones, GHB/GBL and synthetic cannabinoids account for most mentions, and in Northern Ireland the main groups are synthetic cathinones and “other” (mostly 4,4′‐DMAR), especially in 2014 and 2015. These differences are also underlined by looking at the proportions of NPS accounted for by the different groups, eg, benzodiazepine analogues accounted for 94.53% of all Scottish NPS‐related poisoning deaths registered in 2013‐2018, compared to 46.55% by “other” and 44.83% by synthetic cathinones in Northern Ireland in 2013‐2016, and 27.460% by synthetic cathinones, 24.30% by GHB/GBL and 21.30% by synthetic cannabinoids in England and Wales in 2013‐2018.
Table 4.
Number of poisoning deaths involving NPS by selected drug group, country and year, UK, 2013‐2018
| Year | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2013‐2018 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | England and Wales | Northern Ireland | Scotland | UK | England and Wales | Northern Ireland | Scotland | UK | England and Wales | Northern Ireland | Scotland | UK | England and Wales | Northern Ireland | Scotland | UK | England and Wales | Northern Ireland | Scotland | UK | England and Wales | Northern Ireland | Scotland | UK | England and Wales | Northern Ireland | Scotland | UK |
| Drug group | ||||||||||||||||||||||||||||
| Cathinones | 26 | 3 | 4 | 33 | 27 | 8 | 4 | 39 | 49 | 7 | 6 | 62 | 31 | 4 | 1 | 36 | 7 | 4 | 0 | 11 | 16 | 0 | 16 | 156 | 26 | 15 | 197 | |
| Of which, mephedrone | 18 | 0 | 1 | 19 | 22 | 0 | 3 | 25 | 44 | 0 | 2 | 46 | 15 | 0 | 0 | 15 | 1 | 0 | 1 | 2 | 0 | 2 | 102 | 0 | 6 | 108 | ||
| GHB/GBL | 18 | 0 | 0 | 18 | 20 | 1 | 0 | 21 | 26 | 0 | 1 | 27 | 30 | 1 | 0 | 31 | 17 | 0 | 17 | 27 | 0 | 27 | 138 | 2 | 1 | 141 | ||
| Benzodiazepine analogues1 | 3 | 1 | 40 | 44 | 14 | 2 | 44 | 60 | 11 | 1 | 57 | 69 | 10 | 2 | 278 | 290 | 9 | 336 | 345 | 9 | 575 | 584 | 56 | 6 | 1330 | 1392 | ||
| Methiopropamine (MPA) | 4 | 0 | 2 | 6 | 7 | 0 | 5 | 12 | 6 | 0 | 4 | 10 | 10 | 0 | 0 | 10 | 0 | 0 | 0 | 1 | 0 | 1 | 28 | 0 | 11 | 39 | ||
| Alpha‐methyltryptamine (AMT) | 7 | 0 | 3 | 10 | 6 | 0 | 1 | 7 | 5 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 0 | 4 | 22 | ||
| Benzofurans (e.g. 6‐APB) | 7 | 0 | 2 | 9 | 5 | 0 | 0 | 5 | 2 | 0 | 0 | 2 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 2 | 19 | ||
| Novel amphetamines (PMA/PMMA) | 3 | 1 | 9 | 13 | 4 | 4 | 1 | 9 | 1 | 2 | 2 | 5 | 1 | 1 | 2 | 4 | 0 | 0 | 0 | 3 | 1 | 4 | 12 | 8 | 15 | 35 | ||
| Novel opioids | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 5 | 2 | 1 | 0 | 3 | 3 | 0 | 0 | 3 | 2 | 0 | 2 | 11 | 0 | 11 | 22 | 1 | 1 | 24 | ||
| Piperazine derivatives | 1 | 0 | 8 | 9 | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 11 | 14 | ||
| NBOMes | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | ||
| Synthetic cannabinoids | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 3 | 8 | 1 | 0 | 9 | 27 | 2 | 2 | 31 | 24 | 0 | 24 | 60 | 1 | 61 | 121 | 3 | 4 | 128 | ||
| Other NPS2 | 5 | 0 | 2 | 7 | 5 | 15 | 11 | 31 | 14 | 9 | 5 | 28 | 19 | 3 | 4 | 26 | 2 | 1 | 3 | 0 | 1 | 1 | 45 | 27 | 24 | 96 | ||
| Total from deaths examined | 63 | 4 | 60 | 127 | 82 | 22 | 62 | 166 | 114 | 18 | 74 | 206 | 123 | 10 | 286 | 419 | 61 | 4 | 337 | 402 | 125 | 588 | 713 | 568 | 58 | 1636 | 2262 | |
Note 1. These include alprazolam, delorazepam, diclazepam, etizolam, flubromazepam, phenazepam, etc.
Note 2. These include other substances not included in the categories; these vary between countries and over time.
This brief exploration of data from UK GMRs illustrates how such empirical information can identify that priorities for clinicians and policy‐makers vary between parts of the UK over time and between NPS groups, how numbers of NPS‐related poisoning deaths being registered are rising at different rates and to what extent, and how the impact of legislation can be monitored using such epidemiological information. However, differences in approach to defining NPS needs to be borne in mind when interpreting such findings, as well as the other caveats mentioned above.
Typically, serious and fatal outcomes following administration become evident through emergency department (ED) admissions and/or fatalities. Deaths probably provide the most robust epidemiological metric relating to acute health consequences of NPS consumption.
5. INSIGHTS THAT FATALITIES INVOLVING NPS CAN PROVIDE
Whilst information on the overall number of deaths and trends over time is provided through information collated by the GMRs, that provided by SMRs is much richer in terms of detail and context. This is especially so in respect of key aspects about decedents and those regarding their deaths. In respect of those dying, the key aspects that can be focused on are their socio‐demographics, especially sexual orientation, ethnicity, living arrangements and employment status, known medical history, especially any co‐morbidities (physical and/or mental), medical procedures and prescribing, and substance use history and evolution. All of these can help to identify ‘at risk’ populations and sub‐groups.
Information on deaths can cover events and settings leading up to death, circumstances immediately surrounding the death, including interventions, treatments and medications administered, and forensic evidence about what psychoactive substances may have been taken (and whether voluntarily). Toxicology can provide objective identification of what was taken, whether a sole drug or a combination of substances had been consumed, and if possible their relevant levels. Pathology can help to provide understanding of the mechanisms of deaths from specific NPS classes, as well as of pre‐existing risks such as both known and unknown health conditions/morbidities. The information on death certificates with regard to mechanisms of deaths is very patchy and inconsistent, therefore the far richer information from SMRs can provide some indication of the mechanisms and causes of death. However, without adequate information on what amounts have been consumed of all substances detected, and of their individual toxic and lethal toxicological levels, it is difficult to ascribe death to specific molecules or combinations thereof (for reasons outlined elsewhere in this article).
Clinicians can benefit more rapidly from the in‐depth information on NPS collated and published by SMRs, or cases reported to Toxbase maintained by the National Poisons Information Service (https://www.toxbase.org/) or via the Report Illicit Drug Reactions (RIDR) website maintained by Public Health England (https://report-illicit-drug-reaction.phe.gov.uk/).
6. HOW THESE INSIGHTS CAN BE USED BY CLINICIANS AND POLICY‐MAKERS
There are recognised shortcomings in terms of the extent to which mechanisms of death, as opposed to causes of death, are recorded on death certificates, as well as a paucity of relevant detail, and thus in publications emanating from the GMRs. However, the level of detail available to SMRs via Coroners' and Procurators' Fiscal records, especially post‐mortem and associated histological and toxicological reports, can provide insights into symptomatology, medical interventions and ultimately probable mechanisms of death. Where NPS are involved, such information is often the only insight available on how these substances affect the body and/or interact with other substances.36
Acute NPS toxicity admissions to the ED, whether they respond to treatment or not, can generate information that can be used subsequently by clinicians and those responsible for investigating deaths. Treatments can be mapped onto the empirical data provided by mortality statistics and individual case reports and case series to provide tailored approaches to both the different drugs/drug groups used and the types of users themselves within the wider context of the NPS phenomenon.
Clinicians typically are unaware of what substance(s), including NPS, have been used and which symptoms may relate to which substance(s), therefore they have to focus immediately on the presenting symptoms, trying to ascertain what is suspected to have been taken, but only later will there be any opportunity (if any) to confirm consumed molecules. When it is confirmed that an NPS is responsible for the presenting symptoms, sharing the experience of the symptomatology and treatment administered can assist in the development of relevant treatment approaches and protocols to be used in the future. Clinicians' decision‐making processes can be informed by, eg, taking a complete case‐history of all substance use (prescribed or OTC medications, herbal supplements and NPS), asking questions that will draw out relevant information, recording new forms of symptoms and their combinations, and allowing opportunities to collect information, including forensic evidence and body tissue/fluid samples, on the potential/actual NPS used.21
Information obtained in the ED as outlined above, which may include information on deaths confirmed on arrival or where patients subsequently die, can be harnessed together with the information derived from formal death inquiries, such as inquests, to inform treatment options, which in turn can be collated and made available to clinicians via Toxbase or guidance such the Neptune guidelines.37
Policy‐advisers and policy‐makers/legislators too can benefit from the in‐depth information outlined above that can be provided by SMRs and other researchers with access to up‐to‐date information on DRDs (whether academics and/or forensic providers). Such information regularly feeds into decision‐making processes, typically following formal risk assessment processes at national (eg, the UK's Advisory Council on the Misuse of Drugs [ACMD]), regional (EMCDDA) and international (United Nations Office on Drugs and Crime, UNODC) levels. These risk assessments take a range of factors, including social harms, law enforcement issues, prevalence, etc, into account. However, serious adverse consequences, especially fatalities, probably have a greater influence in the decision‐making process. The importance of in‐depth, up‐to‐date information in undertaking rapid risk assessments is particularly evident if a sudden outbreak of deaths occurs in a defined locality (eg, 4,4′‐DMAR in Northern Ireland), when public safety concerns are foremost in the priorities of public health and law enforcement agencies. At a local level, drug and alcohol‐related deaths advisory groups (or similar bodies) can conduct confidential deaths inquiries; the lessons learned from these can then feed into local decisions ranging from the management of physical health issues to long‐term conditions in substance misuse service users.38, 39, 40, 41, 42
Information from members of the UK EWS such as NPSAD and our own research unit, often in collaboration with each other, have provided information on deaths at all three levels on specific molecules or groups of NPS. This has resulted in policy‐advisers making recommendations, subsequently enacted by legislators, to regulate molecules via Temporary Drug Class Orders (that restrict import and sales), adding substances to the schedules of the Misuse of Drugs Act 1971 and thereby making them illegal at a UK level, or even adding them to the United Nations' Single Convention on Narcotic Drugs of 1961, as amended by the 1972 Protocol, and the Convention on Psychotropic Substances of 1971.
However, the use of legislation may be unnecessary. Once users become aware of serious adverse effects via online discussion fora, word of mouth or health alerts, the use of specific molecules may be very short‐lived; methoxetamine is a good example of this phenomenon.43 Indeed, based on clinical information including deaths, legislation may even be inappropriate; khat is a good example in the UK context.44
Although some molecules have been controlled in the past (eg, mephedrone in 2010), they have become part of the repertoire of substances used on a regular basis rather than disappearing because they became illegal. Some have even argued that making ‘legal highs’ illicit substances may have a contradictory effect45: although it might reduce deaths from a particular molecule, deaths from more potentially dangerous substances could occur instead.
One common concern with the introduction of legislation and/or other forms of regulation to restrict the availability of substances causing deaths is whether it causes displacement and whether such changes become permanent. The introduction of controls on NPS in the UK in April 2010 appears to have had mixed results. Whilst the number of deaths involving piperazines did fall following their change to being controlled substances, the controls introduced at the same time on synthetic cathinones, specifically on mephedrone and related derivatives, led to a reduction in mephedrone deaths but also to a search for other non‐controlled cathinones which could act as alternatives to those molecules now caught by the Misuse of Drugs Act 1971. In turn, this may have caused deaths from other synthetic cathinones. Deaths involving mephedrone have reduced but still occur. Whilst regulation and control can have an impact, the provision of unbiased, evidence‐based information and education is also necessary to reduce deaths from all drugs, including NPS.
7. CURRENT NEEDS AND RECOMMENDATIONS
There are several immediate needs that must be addressed to assist epidemiologists, clinicians and policy‐makers concerned with NPS in their complementary roles.
Clearly, to get a more complete understanding of the phenomenon regarding specific NPS molecules or classes of new drugs, it is necessary to try and get data on other dimensions (availability, prevalence, confiscations, etc), but in the early stages of a drug's emergence onto the market such information, if it exists, is largely anecdotal in nature. Therefore, information on fatalities can and does play a key role in the consideration of whether any response is required and, if so, what form it might take. Information generated from NPS‐related deaths can assist in filling the knowledge gaps in respect of mode of action, adverse effects, toxicity and lethality.
More detailed information, timely identification and reporting of NPS‐related fatalities to SMRs is needed. This process may be improved by the introduction of the new medical examiner approach to investigating deaths currently being introduced in England and Wales.46 However, the information still needs channelling to SMRs so that they can collate and publish information quickly. SMRs collect more in‐depth data which are important for newly emerging NPS drug classes, as well as for events having low prevalence, in order to obtain knowledge and understanding of presenting symptoms prior to death and the actual mechanisms of death. This requires more investment in terms of resourcing so that existing expertise is not lost; it would be very difficult to resurrect existing networks and goodwill on which the voluntary reporting systems depend. Funding is an issue for policy‐makers to consider; GMRs and SMRs need proper resourcing and support to continue their contribution to monitoring and provision of advice to Government agencies, the ACMD, etc on DRDs, including NPS.
Investigative procedures used by the different stake‐holders involved in dealing with DRDs need to become uniform and consistent across agencies, both within the UK and elsewhere. Standards could be developed in respect of capturing information about events leading up to and surrounding death, including past substance use, medication and medical histories.
There is a continuing need to carry out investigations into the pharmacology, pharmacodynamics and pharmacokinetics of molecules, especially synthetic cannabinoids and synthetic cathinones. Particularly important is the need to understand the toxicological threats posed by these synthetic substances, whether on their own, in combination with other molecules of the same chemical class or in combination with both other consumed recreational drugs and/or adulterants. This requirement to deepen the evidence base is underpinned by the continuing, but under‐estimated, number of fatalities attributed to these molecules. Indeed, in some parts of the UK, NPS are now accounting for nearly half of all drug‐poisoning related deaths, eg, 45% (588/1313) of such deaths registered during 2018 in Scotland, accounting for 10.4% in Great Britain (England, Wales and Scotland) overall. Indeed, the ‘designer’ benzodiazepine etizolam was implicated in 10.2% (577/5672) of all drug‐poisoning related deaths in 2018.
Deepening the evidence base is facilitated by exchange of information at an international level, which could include national or international guidelines on analytical laboratory standards, standardised lists of molecules to screen for, and sharing mass spectrometry libraries of new analytes and reference standards between institutions.21 Belonging to international agencies, such as the EMCDDA, makes this process easier and more effective as there are recognised channels of communication and understanding.
The accurate completion of death certificates needs to improve, especially with regard to the names of substances implicated in the cause of death, not only in the UK but internationally. To overcome such shortcomings, consideration should be given to including toxicological results on the death certificate only where they have made a pathological contribution47 and stating the generic names of the chemicals causing death.48 In turn, this posits the need for mortality registers to be provided with detailed toxicological and pathological data on a routine basis.
Collaborating and agreeing on approaches to defining NPS and NPS‐related deaths, how to identify such events more accurately, and what statistics and other information regarding them is very important. There needs to be stronger communication between data suppliers (such as Coroners, Medical Examines and Procurators Fiscal) and both GMRs and, more particularly, SMRs.
At a UK level there has been a convergence of approaches to defining what constitutes an NPS, but there is still no single definition used in respect of the publication of poisoning deaths involving NPS. Until the time that such an approach is adopted, there is a need for information on the actual time‐period during which specific molecules were regarded as being NPS to be presented in mortality statistics, especially when breakdowns by specific molecules are not provided. Equally, the need to monitor specific molecules does not disappear when they become controlled, so mortality statistics should continue to report on them. The lead author is aware that GMRs and SMRs within the UK maintain such metadata, so theoretically that information can be and is provided (eg, by NRS).
The publication by GMRs of drug‐related poisoning deaths involving specific molecules is a basic pre‐requisite for substance epidemiologists. Details of whether a substance was implicated on its own or in combination with other substances is a key need, especially where NPS are concerned. Whilst ICD‐1149 (likely to come into use in 2022) contains important improvements in providing new codes for two specific NPS groups,50 synthetic cannabinoids (6C42) and synthetic cathinones (6C47), it does not provide for the addition of any new groups. Indeed, the synthetic cathinones group overlaps with another covering stimulants, including methcathinone (6C46). Thus, as with ICD‐10, there is no provision for identifying specific molecules simply based on ICD codes; a need for attributing deaths to specific molecules – whether NPS or ‘traditional’ drugs – will continue to exist and therefore necessitate adequate extraction protocols based on text searches.
Knowing if named NPS molecules were the only drugs implicated or not in the cause of death enables us to get a better handle on the contribution to deaths made by them, and their lethality.51 Thus, benzodiazepine analogues have particularly low values in terms of the lethality index, whereas γ‐hydroxybutyrate/γ‐butyrolactone, α‐methyltryptamine, synthetic cannabinoids and benzofurans have a higher fatal toxicity. Comparison of the lethality of different drugs or the relative risk of death is important to help target efforts at control, education, service provision, etc. This comparison is possible, eg, see King and Moffat52, 53 for prescribed drugs, and King and Corkery51 for NPS, related to prevalence, availability, amount used/doses. Part of this puzzle can only be addressed when there is a sufficiently critical mass of deaths. What the minimum threshold for such measurement is debatable. For example, King and Corkery51 used a minimum of 10 deaths (whether a substance was implicated on its own or in combination) as the basis for their lethality index. Perhaps a higher number could be used as a threshold, say 10 deaths where the index molecule is the only one implicated in fatality; this would make inferences about toxicity more robust but take longer to accumulate.
At a more fundamental level, we need to understand why different drugs/molecules present with different degrees of toxicity/lethality. This will necessitate, eg, looking at their pharmacology, toxicology and the overlap between the toxicity levels for the same drug and between drugs. Case studies as well as case series and proper surveillance programmes of both intoxications and deaths can contribute to creating a robust evidence base on specific NPS molecules or groups of NPS.
Such an evidence base can help with identification of typologies, eg, ‘at risk’ populations, users' characteristics, groups/classes of drugs consumed together and groups of symptoms/causes of death. In turn, this can inform advice to both policy‐makers and clinicians. The information needs to be tailored to local patterns of drug consumption, which varies both spatially and temporally across the different regions and countries of the UK. Information about NPS deaths in general can be targeted at specific populations, eg, adolescents and young people who typically have a better knowledge and understanding of NPS.54 Such advice needs to be pharmacologically and clinically based. However, the potential problem of ‘closed‐minds’ on the part of policy‐makers needs to be borne in mind in the transmission of such evidence.
It is likely that existing trends will continue but with new features. Thus, polysubstance use with simultaneous consumption of different classes of drugs will persist, but several molecules from the same class (eg, synthetic cathinones) may be taken, some of which may act on different receptors and thus have several different effects. This could lead to difficulties in ascribing specific symptoms to particular molecules. Retailers, purchasers and consumers are still not going to know exactly what is in NPS branded products and even ‘pharmaceuticals’ may contain undisclosed ingredients, eg, currently fake Xanax (Alprazolam) tablets contain Etizolam. There is no reason to suppose that further new synthetic cathinones, synthetic cannabinoids, opioids and benzodiazepines will stop emerging.
Likely new trends include the continued exploration of prescribed medications for recreational purposes, including gabapentinoids, antipsychotics such as olanzapine and quetiapine, promethazine and other antihistamines, and the use of plant‐derived substances to self‐medicate for chronic pain and/or to help with opiate/opioid dependence, including ayahuasca, kratom/mitragynine and iboga/ibogaine/noribogaine. It is therefore important to continue monitoring ED admissions and DRDs closely to identify possible newly emerging and re‐emerging NPS molecules that are causing serious health harms.
8. CONCLUSIONS
Despite the limitations (outlined above) of mortality data in respect of deaths involving NPS, such information can provide insight into how specific molecules may cause or contribute to death, and provide an indicator of their mode of action, toxicity, potential lethality and mechanisms of death, as well as identifying ‘at risk’ populations.
Key to minimising such harms is the timely identification and detailed reporting of such events, followed by their collation and rapid dissemination with sufficient detail to inform clinical approaches and inform policy‐advisers and legislators from a robust evidence base. GMRs and SMRs have complementary roles in this process.
Such scientifically based monitoring data can provide information on the nature and extent of a health risk and possibly its duration, thereby informing public health interventions and service provision, and feeding into policy advice about control and regulation (and whether that would help or hinder). Whether legislation is used or not to restrict the availability and use of particular emerging NPS molecules or groups of molecules, it is important to inform and educate (would‐be) users, clinicians and treatment providers in a timely fashion about the adverse consequences that can occur.
COMPETING INTERESTS
F.S. is a former member of the UK's Advisory Council on the Misuse of Drugs (AMCD) and is on its Novel Psychoactive Substances Committee. J.C. is also on the ACMD's NPS Committee and is a co‐opted member of its Technical Committee, as well as having been a member of the Scottish Government's NPS Expert Group and the UK Focal Point Expert on Drug‐Related Deaths from 2000 to 2015. He was a Home Office official on the ACMD's 1999‐2000 Drug‐Related Deaths Working Group and its recent new working group (2016). J.C. was also a co‐founder of the National Programme on Substance Abuse Deaths in 1997 and was the Programme Manager from 2005 to 2010. The views expressed here are solely those of the authors and do not necessarily reflect those of the ACMD, or past or current employers.
ACKNOWLEDGEMENTS
Thanks to the National Records of Scotland for data provided to the lead author as part of the European Commission‐funded EU‐MADNESS project (Drug Prevention and 2 Information Programme 2014 16; contract no. JUST/2013/DPIP/AG/4823; https://www.eumadness.eu/). The acronym stands for European‐wide, Monitoring, Analysis and knowledge Dissemination on Novel/Emerging pSychoactiveS: integrated EU NPS monitoring and profiling to prevent health harms and update professionals. The aims of the 24‐month project were “to monitor, test, profile, and feed back into education and prevention knowledge relating to the types of NPS emerging in Europe, their associated characteristics and potential harms”. The project had four integrated Workstreams (WS). WS 1 monitored and analysed fatalities and “near‐misses” related to NPS ingestion. Data from these reports informed the choice of compounds investigated in WS 2 and WS 3. WS 2 linked spectral data of established drugs of abuse with their biological data generated by WS 3. WS 3 examined pharmacological and behavioural effects of NPS, which together with information from WS 2 facilitated the development of computational models for recognising and predicting the mode of action, properties and potency of unknown NPS according to their likely chemical structure. WS 4 involved the preparation of educational materials and resources on NPS in a number of European languages on the health risks of NPS for use in inter‐professional medical, pharmacy and health education settings.
Corkery JM, Schifano F, Martinotti G. How deaths can help clinicians and policy‐makers understand the risks of novel psychoactive substances. Br J Clin Pharmacol. 2020;86:482–498. 10.1111/bcp.14183
REFERENCES
- 1. Corkery JM, Orsolini L, Papanti D, Schifano F. Novel psychoactive substances (NPS) and recent scenarios: epidemiological, anthropological and clinical pharmacological issues In: Miolo G, Stair JL, Zloh M, eds. 18 AprilLight in Forensic Science: Issues and Applications. London: Royal Society of Chemistry; 2018a:207‐256, Chapter 8 Print ISBN: 978‐1‐78262‐768‐5 PDF eISBN: 978‐1‐78801‐034‐4 ePub eISBN: 978‐1‐78801‐398‐7 10.1039/9781788010344 http://pubs.rsc.org/en/content/chapter/bk9781782627685-00207/978-1-78262-768-5. [DOI] [Google Scholar]
- 2. Farrell M, Marsden J. Acute risk of drug‐related death among newly released prisoners in England and Wales. Addiction. 2008;103(2):251‐255. PubMed PMID: 18199304. 10.1111/j.1360-0443.2007.02081.x [DOI] [PubMed] [Google Scholar]
- 3. Marsden J, Stillwell G, Jones H, et al. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction. 2017;112(8):1408‐1418. PubMed PMID: 28160345. 10.1111/add.13779 [DOI] [PubMed] [Google Scholar]
- 4. Rychert M, Wilkins C, Parker K, Witten K. Are government‐approved products containing new psychoactive substances perceived to be safer and more socially acceptable than alcohol, tobacco and illegal drugs? Findings from a survey of police arrestees in New Zealand. Drug Alcohol Rev. 2018. Mar;37(3):406‐413. PubMed PMID: 29285812. 10.1111/dar.12655 [DOI] [PubMed] [Google Scholar]
- 5. Rychert M, Wilkins C, Witten K. Issues with monitoring the safety of psychoactive products under a legal regulated market for new psychoactive substances ('legal highs') in New Zealand. Drug Alcohol Rev. 2017. Sep;36(5):589‐596. PubMed PMID: 28229493. 10.1111/dar.12507 [DOI] [PubMed] [Google Scholar]
- 6. Wilkins C. A critical first assessment of the new pre‐market approval regime for new psychoactive substances (NPS) in New Zealand. Addiction. 2014. Oct;109(10):1580‐1586. PubMed PMID: 24529166. 10.1111/add.12484 [DOI] [PubMed] [Google Scholar]
- 7. Shulgin AT, Shulgin LA, Peyton Jacob III, P. A protocol for the evaluation of new psychoactive drugs in man. Meth and Find Exptl Clin Pharmacol. 1986;8(5):313‐320. Available at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.688.3057&rep=rep1&type=pdf Accessed on 26 April 2019. [PubMed] [Google Scholar]
- 8. EMCDDA . European Drug Report 2019: Trends and Developments. European Monitoring Centre for Drugs and Drug Addiction. June, Luxembourg: Publications Office of the European Union. 2019b. ISBN 978‐92‐9497‐398‐6; DOI: 10.2810/191370 http://www.emcdda.europa.eu/publications/edr/trends-developments/2019_en Accessed on 25 August 2019 [DOI]
- 9. Home Office . Drug misuse: findings from the 2017 to 2018 Crime Survey for England and Wales. Home Office Statistical Bulletin 14/18. 26 July. London: Home Office; 2018. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/729249/drug-misuse-2018-hosb1418.pdf https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/728748/drug-misuse-1718-tables.xlsx Accessed on 28 August 2019 [Google Scholar]
- 10. Barratt MJ, Ferris JA, Zahnow R, Palamar JJ, Maier LJ, Winstock AR. Moving on from representativeness: testing the utility of the global drug survey. Subst Abuse. 2017;11:1178221817716391 PubMed PMID: 28924351; PubMed Central PMCID: PMC5595253. 10.1177/1178221817716391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO . ICD‐10: The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. 1 January, Geneva, World Health Organization. 1992. ISBN‐10: 9241544228; ISBN‐13: 978–9241544221. Available online here: https://icd.who.int/browse10/2010/en Accessed on 19 April 2019.
- 12. NRS . Drug‐related Deaths in Scotland in 2017. Edinburgh: National Records of Scotland. 3 July. 2018. Available at: https://www.nrscotland.gov.uk/files//statistics/drug-related-deaths/17/drug-related-deaths-17-pub.pdf Accessed on 21 May 2019
- 13. ONS . Mortality Statistics: Metadata. July. 2015. Available at: http://www.ons.gov.uk/ons/guide-method/user-guidance/health-and-life-events/mortality-metadata.pdf Accessed on 21 May 2019
- 14. Claridge, H. , Goodair, C . (2015). Drug‐related deaths in England, Northern Ireland, The Channel Islands and the Isle of Man: January‐December 2013. National Programme on Substance Abuse Deaths (NPSAD), St George's, University of London. Available at: https://www.sgul.ac.uk/images/NPSAD_-_Drug-related_deaths_in_England_Northern_Ireland_the_Channel_Islands_and_the_Isle_of_Man_January-December_2013.pdf Accessed on 21 May 2019
- 15. ISD Scotland . National Drug‐Related Deaths Database. Edinburgh: Information Services Division Scotland; 2010. Available at: https://www.isdscotland.org/Health-Topics/Drugs-and-Alcohol-Misuse/Drugs-Misuse/Drug-Related-Deaths-Database/ Accessed on 21 May 2019 [Google Scholar]
- 16. Guillou C. New psychoactive substances: challenges of sharing analytical data and knowledge in a European regulatory context. Toxicologie Analytique & Clinique. 2017;29(1):1‐2. https://www.em-consulte.com/article/1105203/article/new-psychoactive-substances-challenges-of-sharing- [Google Scholar]
- 17. Hikin L, Smith PR, Ringland E, Hudson S, Morley SR. Multiple fatalities in the north of England associated with synthetic fentanyl analogue exposure: detection and quantitation a case series from early 2017. Forensic Sci Int. 2018;282:179‐183. PubMed PMID: 29216524. 10.1016/j.forsciint.2017.11.036 [DOI] [PubMed] [Google Scholar]
- 18. Sisco E, Verkouteren J, Staymates J, Lawrence J. Rapid detection of fentanyl, fentanyl analogues, and opioids for on‐site or laboratory based drug seizure screening using thermal desorption DART‐MS and ion mobility spectrometry. Forensic Chem. 2017;4:108‐115. 10.1016/j.forc.2017.04.001 PubMed PMID: 29251300; PubMed Central PMCID: PMC5731661. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5731661/ https://www.sciencedirect.com/science/article/pii/S2468170917300152?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mollerup CB, Dalsgaard PW, Mardal M, Linnet K. (2017). Targeted and non‐targeted drug screening in whole blood by UHPLC‐TOF‐MS with data‐independent acquisition. Drug Test Anal. 2017. Jul;9(7):1052‐1061. PubMed PMID: 27750404 10.1002/dta.2120 [DOI] [PubMed] [Google Scholar]
- 20. Ministry of Justice . Coroners Statistics 2018: England and Wales. 9 May. London: Ministry of Justice; 2019. Available at: https://www.gov.uk/government/statistics/coroners-statistics-2018 Accessed on 26 August 2019 [Google Scholar]
- 21. EMCDDA . An analysis of post‐mortem toxicology practices in drug‐related death cases in Europe, Technical report. European Monitoring Centre for Drugs and Drug Addiction. April, Luxembourg: Publications Office of the European Union. 2019a. ISBN: 978‐92‐9497‐408‐2; DOI: 10.2810/81554 http://www.emcdda.europa.eu/system/files/publications/10544/Analysis%20of%20practices%20of%20PM%20toxicology%20of%20DRD%20in%20Europe_EMCDDA%20Technical%20report.pdf Accessed on 29 April 2019 [DOI]
- 22. Société Française de Toxicologie Analytique . Recommandations de la SFTA pour la réalisation des analyses toxicologiques dans les cas de décès impliquant des NPS [SFTA guidelines for the achievement of toxicological analyzes for deaths involving NPS]. Toxicologie Analytique & Clinique. 2017;30(1):1‐4. 10.1016/j.toxac.2017.07.002 [DOI] [Google Scholar]
- 23. Corkery JM, Goodair C, Claridge H. Synthetic cathinones and related fatalities in the United Kingdom. Chapter 11 In: Corazza O, Roman‐Urrestarazu A, eds. Handbook of Novel Psychoactive Substances – What Clinicians Should Know about NPS. London: Routledge; 2018b:185‐210 ISBNs: Paperback: 9781138068308; Hardback: 9781138068292 https://www.routledge.com/Handbook-of-Novel-Psychoactive-Substances-What-Clinicians-Should-Know/Corazza-Roman-Urrestarazu/p/book/9781138068308. [Google Scholar]
- 24. Comstock GW, Markush RE. Further comments on problems in death certification. Am J Epidemiol. 1986. Aug;124(2):180‐181. PubMed PMID: https://www.ncbi.nlm.nih.gov/pubmed/3728435. 10.1093/oxfordjournals.aje.a114376 [DOI] [PubMed] [Google Scholar]
- 25. Ermenc B. Comparison of the clinical and post mortem diagnoses of the causes of death. Forensic Sci Int. 2000;114(2):117‐119. PubMed PMID: 10967252. 10.1016/S0379-0738(00)00329-7 [DOI] [PubMed] [Google Scholar]
- 26. ONS . Deaths related to drug poisoning in England and Wales QMI. 9 March. Newport, Gwent: Office for National Statistics; 2018. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/methodologies/deathsrelatedtodrugpoisoninginenglandandwalesqmi Accessed on 25 May 2019 [Google Scholar]
- 27. Buchanich JM, Balmert LC, Williams KE, Burke DS. The effect of incomplete death certificates on estimates of unintentional opioid‐related overdose deaths in the United States, 1999‐2015. Public Health Rep. 2018;33(4):423‐431. PubMed PMID: https://www.ncbi.nlm.nih.gov/pubmed/29945473; PubMed Central PMCID: https://www.ncbi.nlm.nih.gov/pubmed/PMC6055296. 10.1177/0033354918774330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wood DM, Conran P, Dargan PI. ICD‐10 coding: poor identification of recreational drug presentations to a large emergency department. Emerg Med J. 2011;28(5):387‐389. PubMed PMID: 20660895. 10.1136/emj.2009.088344 [DOI] [PubMed] [Google Scholar]
- 29. Wood DM, De La Rue L, Hosin AA, et al. Poor identification of emergency department acute recreational drug toxicity presentations using routine hospital coding systems: the experience in Denmark, Switzerland and the UK. J Med Toxicol. 2019;15(2):112‐120. PubMed PMID: 30603897; PubMed Central PMCID: PMC6440929. 10.1007/s13181-018-0687-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corkery J. UK drug‐related mortality – issues in definition and classification. Drugs and Alcohol TodayJun. 2008;8(2):17‐25. 10.1108/17459265200800014 [DOI] [Google Scholar]
- 31. Goodair CM, Corkery J, Claridge H. Legal highs: a problem of definitions? Letter. Lancet. 17 May. 2014;383(9930):1715 PubMed PMID: 24835607. 10.1016/S0140-6736(14)60822-9 [DOI] [PubMed] [Google Scholar]
- 32. NRS . Drug‐related Deaths in Scotland in 2018. Edinburgh: National Records of Scotland. 16 July. 2019. Available at: https://www.nrscotland.gov.uk/files//statistics/drug-related-deaths/2018/drug-related-deaths-18-pub.pdf Accessed on 19 August 2019
- 33. ONS . Deaths related to drug poisoning in England and Wales: 2018 registrations. 15 August. Newport, Gwent: Office for National Statistics. 2019a. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2018registrations/pdf https://www.ons.gov.uk/file?uri=/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsrelatedtodrugpoisoningbyselectedsubstances/2018registrations/2018pivot14082019120833.xlsx https://www.ons.gov.uk/file?uri=/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsrelatedtodrugpoisoningenglandandwalesreferencetable/current/2018drugpoisonings.xlsx Accessed on 19 August 2019
- 34. NISRA . Drug Related and Drug Misuse Deaths 2007‐2017. 4 March. Belfast: Northern Ireland Statistics and Research Agency; 2019. Available at: https://www.nisra.gov.uk/sites/nisra.gov.uk/files/publications/Drug%20Related%20Deaths%20Press%20Release%202017.pdf https://www.nisra.gov.uk/sites/nisra.gov.uk/files/publications/Drug_Tables_17.xls Accessed on 2 June 2019 [Google Scholar]
- 35. ONS . Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland: Mid‐2001 to mid‐2018 detailed time series. 26 June. London: Office for National Statistics. 2019b. Available at: https://www.ons.gov.uk/file?uri=/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatestimeseriesdataset/current/pop.xlsx Accessed on 19 August 2019 [Google Scholar]
- 36. Corkery JM, Streete P, Claridge H, et al. Characteristics of deaths associated with kratom use. J Psychopharmacol. 2019; 33(9):1102‐1123. [Epub ahead of print]. 20 August. PubMed PMID: https://www.ncbi.nlm.nih.gov/pubmed/31429622 10.1177/0269881119862530, 10.1177/0269881119862530 [DOI] [PubMed] [Google Scholar]
- 37. Abdulrahim D, Bowden‐Jones O. Guidance on the Management of Acute and Chronic Harms of Club Drugs and Novel Psychoactive Substances. London: Novel Psychoactive Treatment UK Network (NEPTUNE); 2015. Available at: http://neptune-clinical-guidance.co.uk/wp-content/uploads/2015/03/NEPTUNE-Guidance-March-2015.pdf Accessed on 26 May 2019. [Google Scholar]
- 38. LGA . Preventing drug related deaths: case studies. May. London: Local Government Association: 2017. Available at: https://www.local.gov.uk/sites/default/files/documents/22.3%20Drug%20related%20deaths_v8.pdf Accessed on 31 August 2019 [Google Scholar]
- 39. NTA . Drug‐related deaths: setting up a local review process. London: National Treatment Agency for Substance Misuse; 2011. https://www.bl.uk/britishlibrary/~/media/bl/global/social-welfare/pdfs/non-secure/d/r/u/drugrelated-deaths-setting-up-a-local-review-process.pdf Accessed on 31 August 2019 [Google Scholar]
- 40. PHE . Preventing drug‐related deaths: turning evidence into practice. 1 January. London: Public Health England; 2014. Available at: https://www.gov.uk/government/publications/treating-substance-misuse-and-related-harm-turning-evidence-into-practice/preventing-drug-related-deaths-turning-evidence-into-practice#using-local-reviews-of-drug-related-deaths-to-enhance-provision Accessed on 31 August 2019 [Google Scholar]
- 41. Welsh Assembly Government . Guidance on developing local confidential reviews into drug related deaths in Wales. Cardiff: Welsh Assembly Government. 2005. Available at: https://www.scie-socialcareonline.org.uk/guidance-on-developing-local-confidential-reviews-into-drug-related-deaths-in-wales/r/a11G00000017vK5IAI Accessed on 31 August 2019
- 42. Welsh Government . Consultation document: Guidance for undertaking fatal and non‐fatal drug poisoning reviews in Wales. 23 September. Cardiff: Welsh Government. 2013. Available at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=13&ved=2ahUKEwjR9or_k6_kAhWNa8AKHTcUByQ4ChAWMAJ6BAgAEAI&url=https%3A%2F%2Fico.org.uk%2Fmedia%2Fabout-the-ico%2Fconsultation-responses%2F2013%2F2143%2Fdrug-poisoning-consultation-document-20130923.pdf&usg=AOvVaw3q_LZ2yn_6LRdKIYP98xXY Accessed on 31 August 2019
- 43. Chiappini S, Claridge H, Corkery JM, Goodair C, Loi B, Schifano F. Methoxetamine‐related deaths in the UK: an overview. Hum PsychopharmacolPubMed PMID: https://www.ncbi.nlm.nih.gov/pubmed/26216557. 10.1002/hup.2422. 2015. 10.1002/hup.2422 [DOI] [PubMed] [Google Scholar]
- 44. Corkery JM. Khat – chewing it over: continuing ‘cultural cement’, cardiac challenge or catalyst for change? In: Davies S, Johnston A, Holt D, eds. Forensic Toxicology – Drug Use and Misuse14 July. London: Royal Society of Chemistry; 2016:165‐207 ISBN: 9781782621560. http://pubs.rsc.org/en/content/ebook/978-1-78262-156-0#!divbookcontent. [Google Scholar]
- 45. Bird S. Banned drug may have saved lives, not cost them. 22 November. Straight statistics. 2010. Available at: https://straightstatistics.fullfact.org/article/banned-drug-may-have-saved-lives-not-cost-them Accessed on 26 May 2019
- 46. Luce T, Smith J. Death certification reform in England. BMJ. 2018;361:k2668 PubMed PMID: 29930196. 10.1136/bmj.k2668 [DOI] [PubMed] [Google Scholar]
- 47. Gill JR, Stajíc M. Classical mistakes in forensic toxicology made by forensic pathologists. Academic Forensic Pathology. 2012;2(3):228‐234. 10.23907/2012.034 [DOI] [Google Scholar]
- 48. Department of Health and Human Services, Centers for Disease Control and Prevention, National Centre for Health Statistics (2003). Medical examiners' and coroners' handbook on death registration and fetal death reporting, Hyattsville, MD. Available at: http://unstats.un.org/unsd/vitalstatkb/ExportPDF50262.aspx Accessed on 25 May 2019
- 49. WHO . ICD‐11 for Mortality and Morbidity Statistics (ICD‐11‐MMS). 18 June, Geneva, World Health Organization, 2018. Available at: https://icd.who.int/browse11/l-m/en Accessed on 26 May 2019. [Google Scholar]
- 50. Poznyak V, Reed GM, Medina‐Mora ME. Aligning the ICD‐11 classification of disorders due to substance use with global service needs. Epidemiol Psychiatr Sci. 2017;27(3):212‐218. PubMed PMID: 29198240. 10.1017/S2045796017000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. King LA, Corkery JM. An index of fatal toxicity for new psychoactive substances. J Psychopharmacol. 2018;32(7):793‐801. PubMed PMID: 29482434. 10.1177/0269881118754709 [DOI] [PubMed] [Google Scholar]
- 52. King LA, Moffat AC. Hypnotics and sedatives: an index of fatal toxicity. Lancet. 1981;1(8216):387‐388. PubMed PMID: 6110021. 10.1016/S0140-6736(81)91709-8 [DOI] [PubMed] [Google Scholar]
- 53. King LA, Moffat AC. A possible index of fatal drug toxicity in humans. Med Sci Law. 1983;23(3):193‐198. PubMed PMID: 6633207. 10.1177/002580248302300307 [DOI] [PubMed] [Google Scholar]
- 54. Martinotti G, Lupi M, Carlucci L, et al. Novel psychoactive substances: use and knowledge among adolescents and young adults in urban and rural areas. Hum Psychopharmacol. 2015;30(4):295‐301. PubMed PMID: 26216566. 10.1002/hup.2486 [DOI] [PubMed] [Google Scholar]


