Figure 1.
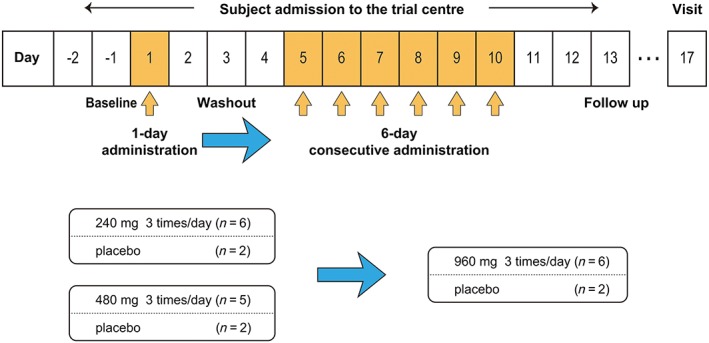
Study design. All subjects were admitted to the trial centre from day −2 (evening) until day 13 (morning). The orange arrow indicates 3 times daily administration of the study drug. Administration of 240 and 480 mg of the study drug was conducted in parallel. The blue arrow indicates a safety examination performed to determine the further progress of the study
