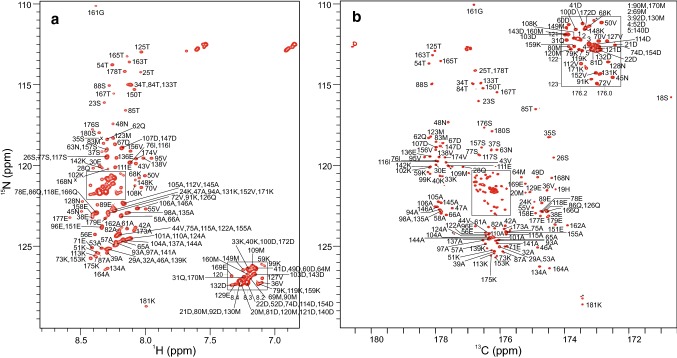
Fig. 2.
a 2D 1H, 15N-HSQC spectrum of BilRI. The peaks are labeled with residue numbers and one-letter amino acid codes. Crosses indicate peaks found at lower contour levels. Residue numbering corresponds to that of whole BilRI protein (1–181) although the construct used was shorter (21–181). b 2D CON spectrum of BilRI. The peaks are labeled with residue number and amino acid code of the amide nitrogen in the C′-NH pair. The peak of the only proline of the BilRI amino acid sequence, which resonates at 172.8 (26Ser C′), 138.1 (27Pro NH) ppm is not shown. Asterisks indicate impurities. Both 2D spectra were acquired at 800 MHz 1H frequency, 25 °C from a 1 mM BilRI sample at pH 6.5