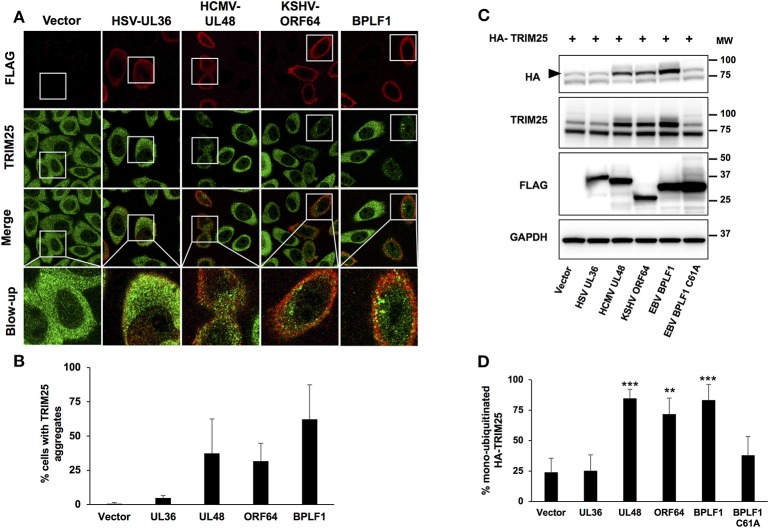
Figure 1.
TRIM25 aggregate formation and autoubiquitination in cells expressing the viral ubiquitin deconjugases. HeLa cells were transfected with FLAG-tagged versions of the viral enzymes and cells were harvested 24 h after transfection. (A) Representative micrographs illustrating the formation of TRIM25 aggregates. Confocal images were obtained with a 63 x lens objective magnification. TRIM25 is green, BPLF1 is red. (B) Quantification of the number of FLAG expressing cells exhibiting TRIM25 aggregates. The mean ± SD of two independent experiments is shown. (C) Representative western blots illustrating the induction of TRIM25 autoubiquitination in cells expressing the viral enzymes. Hela cells were co-transfected with HA-TRIM25 plasmid and plasmids encoding the indicated catalytically active FLAG-tagged N-terminal domains of herpesvirus deconjugases along with catalytically inactive FLAG-BPLF1-C61A. Western blots were probed with the HA antibody. A band shift corresponding to monoubiquitinated TRIM25 (indicated by a black arrow) was detected in cells transfected with BPLF1, UL48, and ORF64 but not in cells transfected with HSV-UL36. The western blots from one representative experiment out of 4 are shown. (D) The intensity of the TRIM25 and the mono-ubiquitinated TRIM25 was quantified by densitometry and the percentage of mono-ubiquitinated TRIM25 was calculated. The means ± SD of 4 experiments are shown. Statistical analysis was performed using the Student's t-test: **P ≤ 0.01 and ***P ≤ 0.001.