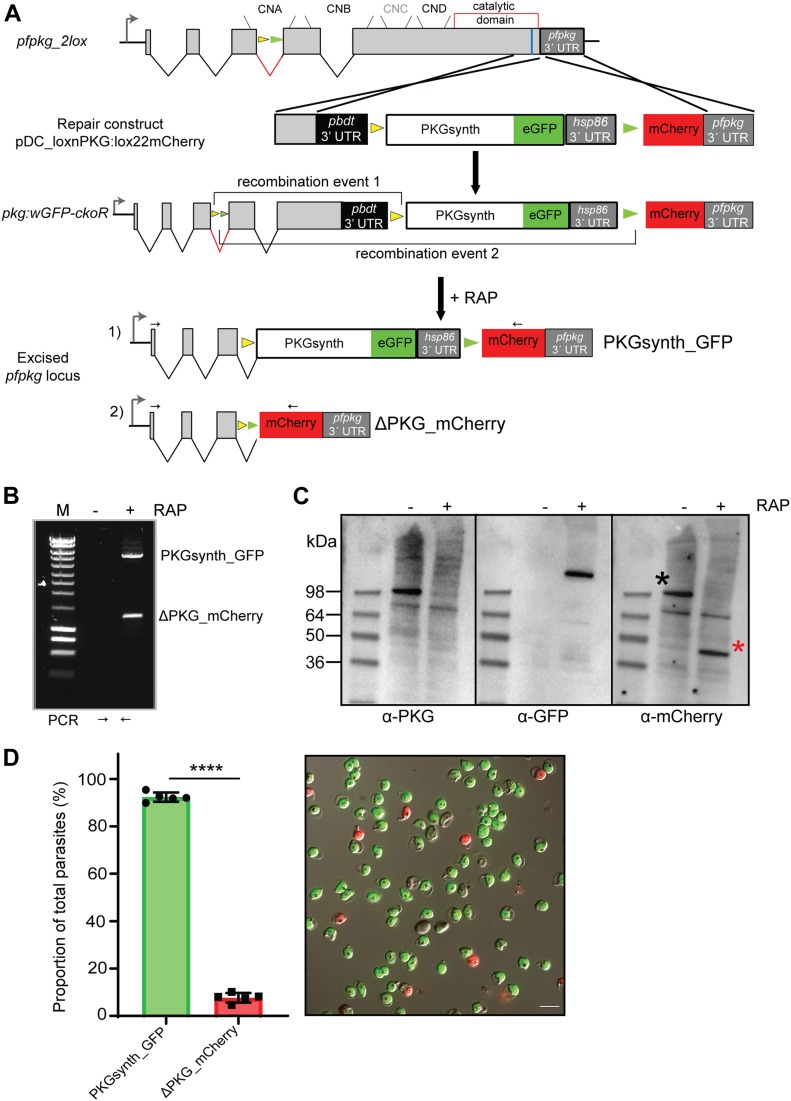
Figure 2. Stochastic recombination between multiple lox sites creates genetically distinct parasite populations within a single culture.
(A) Schematic of the Cas9-enhanced targeted homologous recombination approach used to create the pkg:wGFP-ckoR line. Positions of the four consensus cyclic nucleotide-binding domains (CNA-CND) and the catalytic domain (open red box) of PKG are shown. The position targeted by the guide RNA used to direct Cas9-mediated cleavage is indicated (blue line), as are positions of the loxN (yellow arrowheads) and lox2272 (green arrowheads) sites. RAP-induced DiCre activity switches expression from wt PKG to either a gene replacement with a partially synthetic pfpkg gene fused to eGFP (recombination event 1; PKGsynth_GFP) or to gene disruption and expression of a truncated protein lacking the cyclic nucleotide-binding and kinase domains, fused to mCherry (recombination event 2; ΔPKG_mCherry). Black arrows; oligonucleotide primers used for identification of both events by diagnostic PCR. Note that tagging of PKG with a C-terminal eGFP was expected to be tolerated because it has previously been achieved in the rodent malaria model P. berghei (29). (B) Diagnostic PCR showing generation of products corresponding to both predicted recombination events upon amplification from genomic DNA of RAP-treated pkg:wGFP-ckoR parasites. Expected sizes of the amplicons corresponding to PKGsynth_GFP and ΔPKG_mCherry are 5.6 and 1.3 kb, respectively. Multiple attempts to amplify the corresponding region from mock-treated parasites (−RAP) failed, probably because of the large size of the predicted amplicon (∼10 kb). (C) Western blot showing loss of expression of PKG upon RAP treatment of pkg:wGFP-ckoR parasites, concomitant with appearance of signals corresponding to the PKG_GFP fusion (expected molecular mass 125 kD) and the mCherry fusion (expected mass 38 kD; red asterisk). After being probed with rabbit anti-PKG antibodies (left-hand blot), the membrane was re-probed with a rabbit anti-mCherry antibody to demonstrate the appearance of mCherry only in the RAP-treated sample and to highlight the difference between the DMSO- and the RAP-treated samples for both PKG (black asterisk) and mCherry. Note that the PKG_GFP fusion is not recognised by the commercially available PKG antibody because of masking of the C-terminal epitope, as previously observed (57). (D) (Left) Quantification by flow cytometry of the relative proportions of PKGsynth_GFP and ΔPKG_mCherry parasites in RAP-treated pkg:wGFP-ckoR parasites (sampled at the end of cycle 0). Individual values (dark circles or squares) are shown from five biological replicate experiments (n = 5), and mean values are indicated as bars. Statistical significance was determined by unpaired t test (P-value < 0.0001). (Right) Representative image from differential inference contrast/fluorescence microscopic examination of RAP-treated pkg:wGFP-ckoR parasites (end of cycle 0), showing both GPF- and mCherry-positive schizonts. Scale bar, 10 μm.