Abstract
The capacity of human umbilical cord mesenchymal stem cells (hUMSCs) for migration and homing is very important in regenerative medicine. A detoxified derivative of lipopolysaccharides (LPS) that lacks many of the endotoxic properties of LPS is monophosphoryl lipid A (MPLA). Effects of MPLA on the induction of MSCs migration, have not yet been studied. Also, studies have shown that supernatant of Lactobacillus acidophilus (SLA) culture medium, can stimulate the proliferation of macrophages and lymphocytes in vitro by affecting the properties of the chemotaxis and angiogenesis. Our present study aimed to understanding of the migration of hUMSCs during treatment with MPLA and SLA, separately. HUMSCs were isolated from human umbilical cord and were characterized by investigating surface markers (CD105, CD90, anti-CD29, CD45, and CD34) and their differentiation into adipogenic and osteogenic lineages. HUMSCs were treated with MPLA (10-3 µg/ml) and SLA (3 µl/ml). The morphological changes were not observed in both treated MSCs. Expression levels of migration markers were determined by quantitative reverse transcription PCR analysis on 2, 4, 6 days after treatment. Results showed that VEGF and MMP-2 but not CXCR-4 was increased in the presence of both treatments. Also, SLA led to a decrease and increase of the expression of VLA-4 and VCAM-1, respectively, while MPLA increased both VLA-4 and VCAM-1 expression.Therefore, it can be suggested that MPLA has more prominent results than SLA, but both treatments can probably be considered as an inducing factor in migration.
Key Words: Human umbilical cord mesenchymal stem cells, monophosphoryl lipid A, Lactobacillus acidophilus
Mesenchymal stem cells (MSCs) are suitable for cell therapy and regenerative medicine due to their capacity for migration and homing (1, 2). The unsuccessful homing of stem cells will reduce the therapeutic effects, so a vital step in cell therapy is the expression of the genes involved in migration. C-X-C chemokine receptor type 4 (CXCR-4), very late antigen-4 (VLA-4), vascular cell adhesion molecule 1 (VCAM-1), matrix metalloproteinase-2 (MMP-2), and vascular endo-thelial growth factor (VEGF) play an important role in various stages of migration including chemotaxis, rolling, and invasion (2-5). Among various cellular sources of stem cells, umbilical cord is considered as an interesting source which has high benefits (6). Some of these benefits are as below: the greater amount of cells compared with bone marrow (per unit of volume), lower rate of graft-versus-host-disease, no special moral considerations, ease of collection, lower risk of transmission of infectious diseases especially Epstein-Barr virus and cytomegalovirus, no pain for either the mother or the child (7-10). Monophosphoryl lipid A (MPLA) is a well-characterized toll-like receptor 4 (TLR-4) agonist that has been utilized as a vaccine adjuvant in some vaccines that are U.S. FDA approved. MPLA is a detoxified derivative of lipopolysaccharides that lacks many of the endotoxic properties, and yet retains both its adjuvant and immunostimulatory activities. MPLA, commercially available as an immunostimulant, was developed both as an adjuvant for human vaccines and as a prophylactic drug for septic shock (11). It has been used as an adjuvant and immunological system stimulant, but its effects have not been studied on the expression of migration factors in human umbilical cord mesenchymal stem cells (hUMSCs). It is therefore not clear whether MSCs migration can be induced by MPLA. Although cell migration has been widely studied, the mechanisms involved in inducing the migration of MSCs are unknown and the effects of MPLA and bacterial release materials on migration have not been studied so far. One of the most important probiotic bacteria in the digestive system is Lactobacillus acidophilus PTCC 1634. Various studies have shown that this bacterium has a variety of effects on increasing the health, and activating factors of human and animal immunity both in vitro and in vivo (12). Previous studies have shown that supernatant of L. acidophilus affects chemotaxis and angiogenesis properties of cells, and thus can stimulate the proliferation of macrophages and lymphocytes in vitro. Also, it can induce the chemotaxis properties and proliferation of fibroblasts, endothelial cells and inflammatory cells in vivo (13). Since migration action is important for achieving better therapeutic results in clinical setting, selection of suitable MSCs source with high migration capacity is crucial in cell therapy and regenerative medicine. Thus, in the present study we compared the effect of MPLA with bacterial release materials of L. acidophilus on the expression of genes involved in the migration of hUMSCs.
Materials and Methods
Isolation and expansion of hUMSCs
Informed consents were obtained from all patients before sampling. All experiments were approved by the Ethics and Clinical Studies Research Committee of SKUMS according to Helsinki declaration. Informed consents were obtained from all mothers before surgery.
Following umbilical cord collection, hUMSCs were isolated from human umbilical cords according to a protocol published previously (14). Briefly, the umbilical cord samples were washed with sterile PBS solution to remove traces of blood, 10-cm pieces were produced, and the vessels were separated and closed at the two ends with a sterile plastic clamp. They were then treated by collagenase type I enzyme for 3 h. In order to inactivate collagenase activity, the pieces were incubated in Dulbecco’s modified Eagle’s medium–low glucose (DMEM-LG, Life Technologies, USA) containing 10% fetal bovine serum (FBS, Life Technologies, USA).
For identification of surface antigens, the hUMSCs (1×106) were stained with anti-CD105, anti-CD90, anti-CD29, anti-CD45, and anti-CD34 (Abcam, UK). The related isotype control including FITC- and PE-conjugated mouse IgG isotype antibodies was used for negative staining. Following the incubation at room temperature in the dark (20 min), stained cells were resuspended in 500 µl PBS and analyzed by CyFlow® Space flowcytometer (Partec, Germany). HUMSCs have been shown to be multipotent and capable to differentiate into adipogenic and osteogenic lineages. To promote adipogenic differentiation, after seeding cells in six-well plates (2 × 104 cells /well) with complete media reached 90% confluency, media was changed with adipogenic induction medium containing 0.5 μM isobutyl xanthine (Sigma, MI, USA), 50 μM indomethacin (Sigma, MI, USA), 2.0 μM insulin (Sigma, MI, USA), 0.5 μM dexamethasone (Sigma, MI, USA), and 10% FBS. The fresh medium was replaced every 3 days. After 3 weeks, following fixation by 4% paraformaldehyde (Sigma, MI, USA) and washing with PBS, cells were stained with 0.3% Oil red O solution (Sigma, MI, USA) for 30 min at room temperature. On the other hand for osteogenesis, after preparation with 0.1 µM dexamethasone (Sigma, MI, USA), 0.2 µM ascorbic acid 2-phosphate (Sigma, MI, USA), 10 mM glycerol 2-phosphate (Sigma, MI, USA), and 10% FBS, and fixation cells were stained with Alizarin red S (Fluka Buchs SG, Switzerland).
Preparation of MPLA and supernatant of L . acidophilus
The primary stock solution of MPLA was prepared by solving 1 mg MPLA (InvivoGen, USA) powder in 1000 µl DMSO (Merck, Germany). The final concentration of MPLA used for cultured cells was 10-3 µg/ml. L. acidophilus (PTCC 1643) was purchased as a lyophilized powder from Iranian research organization for science and technology (IROST). Briefly, L. acidophilus was cultured in MRS broth medium containing 0.05 L-cysteine under microaerophilic conditions for 5 days. In these conditions, the number of bacteria was about 2.5 x 108 colony forming units. Then, the suspension was centrifuged at 10,000 g for 15 min at 4 °C. The supernatant was filtered through 0.2 µm filter, and stored at -20 ° C for further testing. The amount of supernatant used for cultured cells was 3 µl per 1 ml media.
Treatment of hUMSCs with MPLA and supernatant of L. acidophilus
Approximately 2.5×105 huMSCs per well were cultured in 6-well plates containing DMEM-LG supplemented with 10% of FBS (Life Technologies, USA) and 1% of Pen/Strep. After 24 h, two different treatments including MPLA (Invivogen, USA) and supernatant of L. acidophilus were tested on hUMSCs grown as monolayer cultures. The treated hUMSCs were followed for 2, 4 and 6 days carefully.
RNA isolation, cDNA synthesis, and qRT-PCR
Total RNA was extracted from the hUMSCs using TRizol reagent (Sigma, MI, USA) according to manufacturer’s protocol, and quantified by NanoDrop 2000 Spectrophotometer (Thermo Scientific, MA, USA). The 260/280 and 260/230 values were higher than 1.9. Equal amounts of total isolated RNA (1 µg) per sample were reverse transcribed using a cDNA synthesis kit (Yektatajhizazma [YTA], Tehran, Iran) and transferred into the qRT-PCR reaction. The transcription levels of VCAM-1, MMP-2, VLA-4, CXCR-4 and VEGF were evaluated using transcript-specific primers and SYBR® Green PCR Master Mix (Yektatajhizazma [YTA], Tehran, Iran). Specific cycling parameters in the qRT-PCR included an initial denaturation step at 95 °C for 2 min, denaturation at 95 °C for 10 s, annealing at 61 °C for VCAM-1 and MMP-2, 56 °C for VLA-4 and CXCR-4, 57 °C for VEGF (all for 20 s), followed by an extension step at 72 °C for 25 s. The number of cycles was optimized at 40. The primer sequences used were as follows: VCAM-1, (forward) 5'CGAACCCAAACAAAGGCAGA3' and (reverse) 5'ACAGGATTTTCGGAGCAGGA3 '; MMP-2, (forward) 5'ACCACAGCCAACTACGA TGA3' and (reverse) 5'GCTCCTGAATGCCCTT GATG3'; VLA-4, (forward) 5'TCCAACCTGATC CTGTGTC3' and (reverse) 5'TCGTTGTTCCCATT CACT3'; CXCR-4, (forward) 5'ACCATCTACTC CATCATCTTC3' and (reverse) 5'TGATGACAAA GAGGAGGTC3'; VEGF, (forward) 5'ATCAAACC TCACCAAAGCC3' and (reverse) 5'TCTTTGGT CTGCATTCACATC3'; and GAPDH, (forward) 5'GAGTCCACTGGCGTCTTCAC3' and (reverse) 5'ATGACGAACATGGGGGCA3'. The transcript-tion level of GAPDH was used as an endogenous control. The 2-∆∆Ct method was used to determine the relative expression of genes. QRT-PCR reactions were run using a Rotor-Gene 3000 instrument (Corbett Research, Sydney, Australia).
Statistical analysis
Statistical analyses of the qRT-PCR data were performed using GraphPad statistical software (GraphPad Software, CA, USA). The differences between experimental groups and controls were compared using one-way ANOVA and Tukey post hoc test. All P values <0.05 were considered statistically significant.
Results
Characterization of hUMSCs
It was shown that hUMSCs were capable to differentiate into adipogenic and osteogenic lineages (Fig.1).
Fig. 1.
Differentiation of hUMSCs. A: Oil red O staining after 24 days in adipogenic differentiation medium; B: alizarin red S staining after 24 days in osteogenic differentiation medium
The expression level of VEGF but not CXCR-4 increased in the presence of both MPLA and SLA
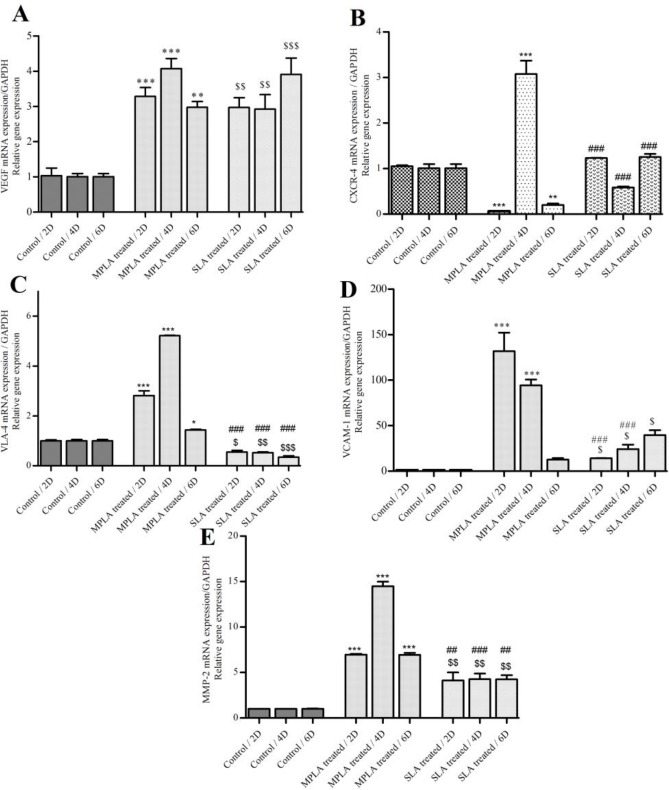
The effect of MPLA on huMSCs migration was examined by investigating mRNA expression level of chemotaxis/ trafficking genes including VEGF and CXCR-4. The qRT-PCR analyzes revealed that the expression of VEGF was up-regulated on the second, fourth, and sixth days after treatment with MPLA and SLA (Fig. 2A). However, the expression of CXCR-4 was different on days after treatment. As seen in figure 2B, after treatment with SLA, the level of CXCR-4 expression was up-regulated and downregulated on the second and fourth days, respectively. Also, after treatment with MPLA the expression of CXCR-4 increased on the fourth day, but declined on the second and sixth days. Upon normalization with the mean expression value, the fold change (15, 16) in VEGF was 3.18, 4.07 and 2.97 on 2, 4 and 6 days after treatment, respectively. In the treatment with SLA on the same days, the fold change values were 2.97, 2.92 and 3.91, respectively (Table 1).
Fig. 2.
QRT-PCR analyzes of genes involved in cell migration. VEGF (A), CXCR-4 (B), VLA-4 (C), VCAM-1 (D) and MMP-2 (E) mRNA expression in hUMSCs after treatment (2D, 4D, 6D) with MPLA and SLA. The graphs show that both treatments led to an increase in expression level of VEGF at all studied times. The data show that under the influence of MPLA, CXCR-4 mRNA expression increased on the fourth day (P = 0.009), and after treatment with SLA, a slight decrease was identified on the fourth day (P = 0.004) as compared with the control. Also, under the influence of MPLA and SLA, VLA-4 mRNA expression increased and decreased on days 2, 4, 6 (P < 0.05), respectively. VCAM-1 mRNA expression increased at all of studied times (P < 0.05) in comparison with the control. MMP-2 mRNA expression was up-regulated in hUMSCs treated with MPLA and SLA at all studied times. Data were normalized to the expression levels of GAPDH in the control. 2 day, 4 day, 6 day. All values are expressed as mean ± SD for 6 days in each group; * MPLA vs control, $ SLA vs control and # MPLA vs SLA
Table 1.
The fold changes obtained for the expression of VEGF, CXCR-4, VLA-4, VCAM-1, and MMP-2
| Treatment | Day Gene | VEGF | CXCR-4 | VLA-4 | VCAM-1 | MMP-2 |
|---|---|---|---|---|---|---|
| MPLA vs.Control | 2 | 3.18 | -0.06 | 2.81 | 131.6 | 6.94 |
| 4 | 4.07 | 3.07 | 5.2 | 94 | 14.47 | |
| 6 | 2.97 | -0.2 | -1.42 | 12.68 | 6.94 | |
| SLA vs.Control | 2 | 2.97 | ns | -0.55 | 14.1 | 4.11 |
| 4 | 2.92 | ns | -0.52 | 24.28 | 4.27 | |
| 6 | 3.91 | ns | -0.34 | 39.73 | 4.23 | |
| MPLA vs.SLA | 2 | ns | -0.04 | 5.1 | 9.35 | 1.67 |
| 4 | ns | 5.29 | 10.03 | 3.88 | 3.38 | |
| 6 | ns | -0.16 | 4.17 | ns | 1.64 |
SLA leads to a decrease and increase in the expression of VLA-4 and VCAM-1 , respectively, but MPLA increased both VLA-4 and VCAM-1 expression
In order to identify the effects of MPLA and SLA on the expression of genes related to the second stage of migration, the two factors integrin and adhesion molecule including VLA-4 and VCAM-1 were evaluated, respectively. The Results of qRT-PCR showed that MPLA and SLA could lead to an increase in the expression of VCAM-1 (Fig.2D), but in the presence of MPLA the expression level of VLA-4 increased. Also, in the presence of SLA the expression of VLA-4 decreased (Fig.2C).
MPLA and SLA lead to increased expression of MMP-2
As the role of MMP-2 which is known as the matrix metalloproteinase in the invasion step of the migration process has been proven (5), in the present study the effects of MPLA and SLA on hUMSCs during the six days after treatment on the expression of MMP-2 were evaluated and the results showed that both MPLA and SLA can increase significantly the expression of MMP-2 (Fig.2E).
Discussion
In this study, we investigated the effect of MPLA and bacterial release materials of L. acidophilus on the expression of migration factors including CXCR-4, VEGF, VLA-4, VCAM-1, and MMP-2 in hUMSCs. The results showed that MPLA had more prominent effects on the increase of the expression of the genes involved in migration in comparison with SLA. Although these effects are much more prominent in the genes involved in the late stages of migration than early stages of migration. Therefore, it can be suggested that the expression levels of genes involved in the early stages of migration (CXCR-4, VLA-4, VEGF) were likely to increase in the first studied time (i.e., before the second day) and then decreased in the next intervals.
Therefore, In the recent studies, the chemoattractive effects of MPLA has been proven (17), and it seems that this reagent may be considered as a stimulator of the migration of MSCs. On the other hand, the effects of probiotics on cell migration have been evaluated (18).
Here, we compared the effects of MPLA as a synthetic reagent and SLA as a probiotic. The results showed that MPLA had more prominent effects on the increase of the expression of the genes involved in migration. Although these effects were much more prominent in the genes involved in the late stages of migration than the genes in the early stages of migration. Therefore, it can be suggested that the expression levels of genes involved in the early stages of migration (CXCR-4, VLA-4, VEGF) were likely to increase in the first studied time (i.e., before the second day) and then decreased in the next intervals. In order to determine the effects of some soluble factors, including chemokines and growth factors, on the potential of MSCs migration in vitro, Ponte et al. showed that among the studied factors, TNF-α led to a significant increase in the migration of bone marrow MSCs. Growth factors such as platelet-derived growth factor-AB (PDGF-AB ) and insulin-like growth factor-1 (IGF-1) are the most susceptible, while the chemokine regulated on activation, normal T cell expressed and secreted (RANTES) and macrophage-derived chemokine (MDC) and stromal-derived factor 1 (SDF-1) had little effect (19). Since in previous studies, VEGF and PDGF have been used as chemoattractants in vitro, and it has been shown that the combination of these two growth factors has a significant effect on MSCs migration (20), in the present study, we evaluated the expression of VEGF, and our findings showed that this growth factor was up-regulated following treatments with MPLA and SLA.
In the present study, the treatments did not have a significant effect on the expression of CXCR-4 in the chemotaxis stage, and only 3.05-fold increase was observed in the treatment with MPLA on the 4th day. Various studies have shown that the CXCR-4/SDF-1 axis plays an important role in the migration of MSCs (21, 22).
As previously mentioned, the effect of MPLA has been investigated on the migration of immune cells, in other words, it has more immunological application. In this study for the first time, the effect of MPLA on MSCs has been investigated, and our findings suggest that it has an important role in the expression and activation of genes involved in the migration. Although it has been shown that MPLA and SLA have remarkable effects on the expression of genes involved in migration, but more studies are needed to determine the mechanism and pathways that affect this process.
In this study, we showed that MPLA can be considered as an effective agent in stimulation of hUMSCs migration, although further studies are needed to optimize the strategy and identify the molecular mechanisms of migration after treatment with MPLA. Probiotics such as L. acidophilus can be considered as effective factors in promoting the migration of hUMSCs, although due to the unknown factors in the solution, it is important to find desirable conditions and concentrations.
Acknowledgements
The authors would like to express sincere thanks to the staffs of Cellular and Molecular Research Center of Basic Health Sciences Institute of Shahrekord University of Medical Sciences (SKUMS) for their cooperation. This research project was part of a Ph.D. thesis and was supported by the deputy of research and technology of Shahrekord University of Medical Sciences [grant number: 3584].
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Heirani-Tabasi A, Hassanzadeh M, Hemmati-Sadeghi S. Mesenchymal stem cells; defining the future of regenerative medicine. Journal of Genes and Cells. 2015;1:34. [Google Scholar]
- 2.Kholodenko IV, Konieva AA, Kholodenko RV. Molecular mechanisms of migration and homing of intravenously transplanted mesenchymal stem cells. J Tissue Eng Regen Med. 2013;2:4. [Google Scholar]
- 3.Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–27. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 4.Ruster B, Gottig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–44. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 5.Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–64. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 6.Azzopardi JI, Blundell R. Umbilical Cord Stem Cells. Stem Cell Discov. 2018;8:1–11. [Google Scholar]
- 7.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 8.Percer B. Umbilical cord blood banking: helping parents make informed choices. Nurs Womens Health. 2009;13:216–23. doi: 10.1111/j.1751-486X.2009.01422.x. [DOI] [PubMed] [Google Scholar]
- 9.Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18:893–908. doi: 10.1016/j.bpobgyn.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Ryan L, Van Syckle K, Coyne KD, et al. Umbilical cord blood banking: procedural and ethical concerns for this new birth option. Pediatr Nurs. 2000;26:105–10. [PubMed] [Google Scholar]
- 11.Salkowski CA, Detore GR, Vogel SN. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect Immun. 1997;65:3239–47. doi: 10.1128/iai.65.8.3239-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganguli K, Walker WA. Probiotics in the prevention of necrotizing enterocolitis. J Clin Gastroenterol. 2011;45 Suppl:S133–8. doi: 10.1097/MCG.0b013e318228b799. [DOI] [PubMed] [Google Scholar]
- 13.Halper J, Leshin LS, Lewis SJ, et al. Wound healing and angiogenic properties of supernatants from Lactobacillus cultures. Exp Biol Med (Maywood) 2003;228:1329–37. doi: 10.1177/153537020322801111. [DOI] [PubMed] [Google Scholar]
- 14.Banitalebi Dehkordi M, Madjd Z, Chaleshtori MH, et al. A Simple, Rapid, and Efficient Method for Isolating Mesenchymal Stem Cells From the Entire Umbilical Cord. Cell Transplant. 2016;25:1287–97. doi: 10.3727/096368915X688911. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 17.Kuai R, Sun X, Yuan W, et al. Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J Control Release. 2018;282:131–9. doi: 10.1016/j.jconrel.2018.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preidis GA, Saulnier DM, Blutt SE, et al. Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. FASEB J. 2012;26:1960–9. doi: 10.1096/fj.10-177980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–45. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 20.Spaeth E, Klopp A, Dembinski J, et al. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–8. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 21.Otsuru S, Tamai K, Yamazaki T, et al. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–34. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 22.Rapp AE, Bindl R, Heilmann A, et al. Systemic mesenchymal stem cell administration enhances bone formation in fracture repair but not load-induced bone formation. Eur Cell Mater. 2015;29:22–34. doi: 10.22203/ecm.v029a02. [DOI] [PubMed] [Google Scholar]