Abstract
BACKGROUND
Multi-phase computed tomography (CT) or magnetic resonance imaging (MRI) has been the standard of care for hepatocellular carcinoma (HCC) diagnosis for years.
CASE SUMMARY
We report a case series of four patients in whom positron emission tomography-computed tomography (PET-CT) scan complemented the conventional CT/MRI scans in evaluating treatment response. In these four cases the conventional multi-phase CT and MRI failed to identify residual HCC disease post-treatment, while PET-CT complemented and aided in treatment response evaluation. In each case, the addition of PET-CT identified and located residual HCC disease, allowed retreatment, and altered medical management.
CONCLUSION
This case series suggests that PET-CT should perhaps play a role in the HCC management algorithm, in addition to the conventional contrast-enhanced multi-phase scans.
Keywords: Hepatocellular carcinoma, Positron emission tomography, Contrast-enhanced multiphase scan, Cirrhosis, Residual cancer, Treatment response evaluation, Case series
Core tip: This is a case series of four hepatocellular carcinoma patients who had undergone locoregional therapies. The conventional multi-phase computed tomography and magnetic resonance imaging scans failed to identify residual hepatocellular carcinoma disease post-treatment, while positron emission tomography-computed tomography scan complemented in treatment response evaluation by identifying and locating residual disease, allowing retreatment.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a well-known complication of chronic liver disease and cirrhosis. It has remained as one of the leading causes of death worldwide[1], responsible for nearly 746000 deaths in 2012[1]. It is the second most common cause of death from cancer globally[1,2]. The incidence of HCC in the United States has been rising in the past four decades[3-5]. Multi-phase computed tomography (CT) or magnetic resonance imaging (MRI) has been the standard of care for HCC diagnosis for years[6]. HCC lesions are known to display arterial enhancement and delayed washout on multi-phase CT or MRI[7]. These contrast-enhanced multi-phase cross-sectional imaging modalities have also been utilized for follow-up on known cases of HCC, especially in determining the response to treatment[8]. Positron emission tomography (PET) scan has been considered unreliable as an imaging modality for HCC diagnosis and for treatment response follow-up due to its lack of sensitivity[9,10]. Many HCC tumors do not show up on PET scan[11]. This case series intends to describe cases in which PET scan complemented the conventional multi-phase CT or MRI in evaluating treatment response.
CASE PRESENTATION
Chief complaints
(1) Case 1: A 62-year-old male with known hepatitis C cirrhosis self-referred to our liver center for further management; (2) Case 2: A 69-year-old male with cryptogenic cirrhosis was referred to our liver center with a 3.3 cm liver lesion in segment 6/7 that appeared to be hypodense without enhancement on a multi-phase CT scan; (3) Case 3: A 62-year-old male with compensated cirrhosis secondary to chronic hepatitis C was referred to our center with HCC tumors based on outside MRI; and (4) Case 4: A 75-year-old female with chronic hepatitis C and compensated cirrhosis was referred to our center due to two HCC tumors, 8.4 cm and 1.2 cm based on multi-phase MRI.
History of present illness
(1) Case 1: The patient was discovered to have HCC upon routine surveillance multi-phase CT, with original tumor burden of 4.2 cm in segment 3 cm and 2.6 cm in segment 5/6. He then received multiple trans-arterial chemo-embolization (TACE) treatments to both lobes of the liver; (2) Case 2: Our multi-disciplinary liver tumor board subsequently reviewed the outside CT scan and confirmed the findings of a non-enhancing hypodense liver lesion. Alpha fetoprotein (AFP) was less than 10 ng/mL. The tumor board recommended a biopsy, which revealed poorly differentiated HCC, based on histological characteristics and immunohistochemical staining. The patient underwent TACE; (3) Case 3: The patient was subsequently treated for HCC tumors (2.1 cm in segment 8, and 1.8 cm and 1.2 cm in segment 6) with two TACE treatments and one microwave ablation (MWA). He was also listed for liver transplant, and a PET/CT scan was done to rule out lung metastasis. He had had tuberculosis, successfully treated many years ago; a chest CT had shown a cavitary lesion within some infiltrate in the right upper lung; and (4) Case 4: The patient’s AFP remained normal in the single digit (ng/mL) at baseline. She subsequently underwent TACE and proton treatments as recommended by our tumor board. The patient underwent multi-phase MRI for monitoring treatment response every three to four months subsequently, and was deemed in complete response for more than two years after the second treatment with proton. She also underwent hepatitis C treatment successfully and achieved sustained virologic response with negative viral titer more than two years from the end of treatment.
History of past illness
(1) Case 1: Negative for diabetes or cardiac disease; (2) Case 2: Diabetes mellitus type 2, atrial fibrillation, skin cancer, esophageal varices; (3) Case 3: Tuberculosis; and (4) Case 4: Diabetes mellitus type 2, and atrial fibrillation.
Personal and family history
(1) Non-contributory (Case 1, 3, 4); and (2) He was exposed to agent orange in the early 1970s (in his 20s) (Case 2).
Physical examination
Anicteric; abdomen soft, non-distended, and non-tender to palpation; liver not palpable; no asterixis (Case 1, 2, 3, 4).
Laboratory examinations
(1) Case 1: AFP rose to 7344 ng/mL approximately 16 mo after presentation; it raised concerns of extrahepatic metastasis, though recent bone scan and chest CT were both negative; (2) Case 2: The patient’s AFP remained low throughout his course, 9.4 ng/mL at the time of the PET/CT scan; his total bilirubin was mildly elevated 2.3 mg/dL while his albumin remained normal, 3.8 g/dL; international normalized ratio (INR) was 1.2; (3) Case 3: The patient’s AFP remained normal throughout his course, 3.8 ng/mL at the time of the PET/CT scan; his albumin remained normal, 3.8 g/dL, while his total bilirubin was slightly elevated 1.5 mg/dL; INR remained normal 1.1; and (4) Case 4: AFP started to increase about 31 mo after presentation, to 24.7 ng/mL, and later to 75 ng/mL in month 36. Total bilirubin had remained normal 0.3 mg/dL, and so had albumin 3.7 g/dL.
Imaging examinations
Case 1: A PET-CT scan was done to search for metastasis, and it revealed three foci of increased fludeoxyglucose (FDG) activity within the treated area of segment 3 (Figure 1A), while showing no FDG activity in the treated area of segment 5/6; no metastasis was identified. A repeat multi-phase MRI was done concurrently, and it failed to reveal any arterial enhancement in the liver (Figure 1B); (2) Case 2: The one-month post-TACE multi-phase CT scan was again inconclusive, showing a 4.2 cm hypodense lesion (Figure 2A) similar to the pre-TACE CT scan. A PET-CT scan was performed, revealing an FDG avid uptake of 3.5 cm × 3.2 cm in measurement at the same area of the liver (Figure 2B) previously biopsied and treated, consistent with residual HCC; (3) Case 3: The PET/CT scan 16 wk post MWA incidentally showed a small site of localized metabolic activity corresponding to a low-density lesion adjacent to a larger right hepatic lobe mass which demonstrated absent metabolic activity, consistent with residual HCC in the treated segment-6 lesion (Figure 3A); no FDG activity in the lungs or elsewhere. A multi-phase MRI a few weeks prior had revealed focal bleed at the periphery of the treatment zone post MWA without evidence of any viable tumor (Figure 3B); and (4) Case: A multi-phase MRI in month 36 was negative for any arterial enhancement, but concurrently a PET-CT scan in month 36 revealed positive FDG uptake at the periphery of the treated lesion in segment 2/3 (Figure 4).
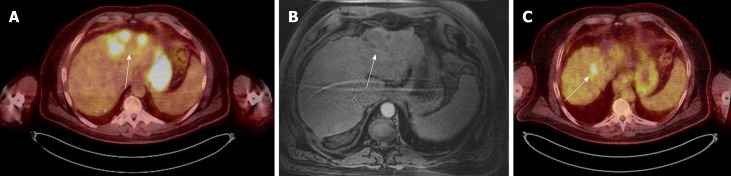
Figure 1.
Case 1. A: Positron emission tomography scan: the white arrow shows the area of multiple foci of fludeoxyglucose uptake in the treated area; B: Multi-phase magnetic resonance imaging scan: it shows no arterial enhancement in the same area during the arterial phase; C: Positron emission tomography scan: the arrow indicates an area of fludeoxyglucose uptake, indicating another residual tumor.
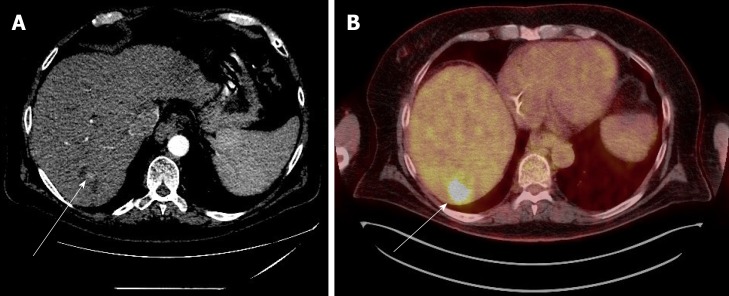
Figure 2.
Case 2. A: Multi-phase computed tomography scan: a hypodense area, indicated by the white arrow, in the liver during the arterial phase post trans-arterial chemo-embolization treatment; B: Positron emission tomography scan: in the same area, there is avid fludeoxyglucose uptake, indicating residual tumor.
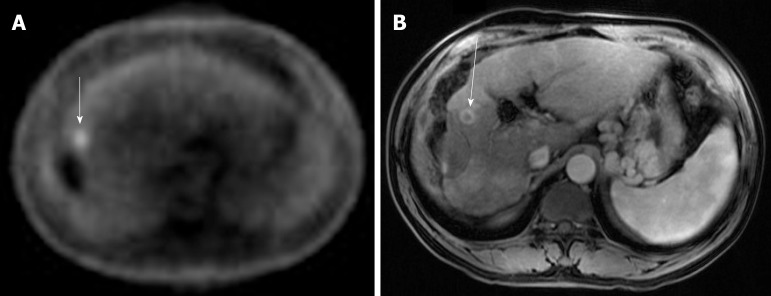
Figure 3.
Case 3. A: Positron emission tomography scan: the white arrow indicates a small focus of fludeoxyglucose uptake adjacent to the treatment zone showing absent metabolic activity; B: Multi-phase magnetic resonance imaging: the white arrow points to focal bleed at the periphery of the treatment zone post microwave ablation without evidence of any viable tumor during the arterial phase.
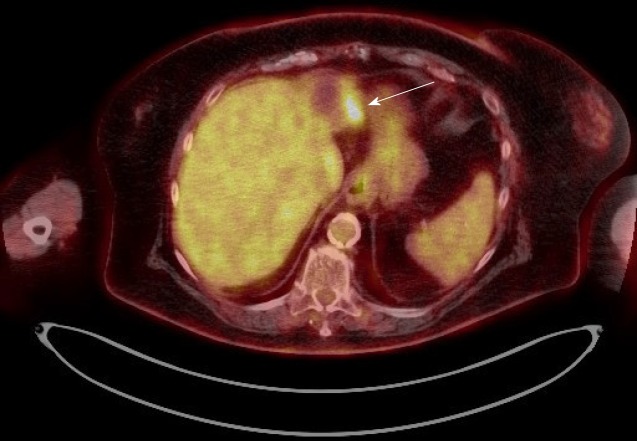
Figure 4.
Case 4. Positron emission tomography scan: the white arrow points to the fludeoxyglucose uptake at the periphery of a treated lesion.
FINAL DIAGNOSIS
Recurrent HCC post locoregional therapies (Case 1, 2, 3, 4).
TREATMENT
Case 1
The patient then underwent more TACE treatments. Both multi-phase MRI scans and PET-CT scans were utilized to monitor treatment response. PET-CT scans subsequently showed residual disease in the left lobe. Treatment modality was changed to proton after the fourth TACE to the segment-3 HCC, approximately 29 mo after presentation.
Case 2
The patient underwent and completed a course of proton treatment consisted of 15 fractions.
Case 3
The patient underwent third TACE approximately five months after the MWA.
Case 4
The patient opted out of recommended laparoscopic ablation, citing her advanced age and the invasiveness of the proposed procedure. She later elected to start nivolumab infusion.
OUTCOME AND FOLLOW-UP
Case 1
The patient’s AFP responded from 3841 ng/mL before the proton treatment to 7 ng/mL after proton. He remained in complete response based on both multi-phase MRI and PET-CT scans every three months until approximately 41 mo after presentation when a new focus of FDG uptake was seen in the dome; a concurrent multi-phase MRI again failed to reveal any arterial enhancement. The dome lesion was treated with proton. Both PET-CT and multi-phase MRI three months post-proton showed the dome lesion well treated, but there was a recurrent HCC focus with arterial enhancement and washout, as well as FDG uptake (Figure 1C), at the previously treated area in segment 6. The patient received proton treatment to segment 6 approximately 48 mo after presentation. The PET-CT scans aided in detecting HCC for this patient and allowed appropriate treatments to prolong his survival. He was followed at our center for a total of 52 mo.
Case 2
The patient was followed up at our center for a total 9 mo. After the proton therapy, he decided to follow up with another institution closer to his residence.
Case 3
Both multi-phase MRI and PET-CT scans one-month post-TACE showed no residual HCC in the liver. The patient was followed up at our center for a total of 37 mo.
Case 4
The patient has tolerated nivolumab infusion well for 14 mo, currently on 2 mg/kg every 2 wk. She has been followed at our center for a total of 58 mo.
DISCUSSION
We have described a series of four cases in which the conventional multi-phase CT and MRI failed to identify residual HCC disease post-treatment, while the FDG PET-CT scan aided in evaluating treatment response (Table 1). In all these cases, FDG PET-CT scans detected residual HCC tumors in treatment zone status post locoregional therapy while the contrast-enhanced multiphase scans could not, and these allowed for timely treatment and meaningful survival.
Table 1.
How positron emission tomography-computed tomography altered medical management in our cases
| Case | Indication for PET-CT | How PET-CT changed management |
| Case 1 | To rule out metastatic HCC while AFP in the 7000s; chest CT and bone scan had been negative | The PET-CT scans successfully detected residual HCC in the treated areas in both lobes and allowed for appropriate treatments to prolong his survival by at least 36 mo; multiple multi-phase MRI scans failed to do so. Subsequently PET-CT scan subsequently detected a new HCC lesion when MRI did not |
| Case 2 | To evaluate treatment response in a biopsy-proven hepatocellular carcinoma mixed with poorly differentiated carcinoma, which had had atypical characteristics on multi-phase CTs (MRI was contraindicated due to his pacemaker) | The PET-CT scan successfully revealed residual carcinoma and allowed for further treatment in prolonging survival |
| Case 3 | To rule out metastatic HCC disease to the lungs in which anatomy had been distorted due to prior Tb infection | The PET-CT scan successfully detected residual HCC while a multi-phase MRI failed to do so. The PET-CT scan subsequently detected a metastatic focus to the bone and averted liver transplant |
| Case 4 | To aid in the investigation of rising AFP in a treated HCC patient when multi-phase MRI scans had been negative | The PET-CT scan identified a recurrent HCC focus in the periphery of a previously treated HCC tumor location |
PET-CT: Positron emission tomography-computed tomography; AFP: Alpha fetoprotein; HCC: Hepatocellular carcinoma; MRI: Magnetic resonance imaging.
Cirrhosis occasionally could alter the vasculature and distort the manifestation of arterial enhancement and delayed washout in HCC tumors via multi-phase CT or MRI, thereby decreasing the sensitivity and specificity of these contrast-enhanced imaging modalities[6,12], not to mention when these HCC tumors have been treated with locoregional therapies or even adjuvant systemic therapy (Case 1). In our case series PET-CT scans appeared very useful when the AFP was elevated and the contrast-enhanced scans did not reveal any pathognomonic findings in treated tumors. The utility and strengths of PET-CT scans are likely underestimated since it is not part of the standard of care in screening for and monitoring HCC, even in the latest United States guidelines[13,14]; it is certainly not part of our institution’s protocol yet. There have been several studies describing the efficacy of combining the traditional 18F-FDG isotope with another isotope, 11C-acetate, in the utility of PET-CT scan in the detection of HCC[15-19]. This dual-tracer approach appears to be quite promising in complementing multi-phase CT or MRI scans, as well as FDG PET-CT scan.
CONCLUSION
PET-CT scans can be very helpful in select HCC cases for monitoring of treatment response, especially when contrast-enhanced multi-phase scans fail to identify pathognomonic findings of residual HCC tumors. A prospective study comparing the addition of dual-tracer PET-CT scan to the conventional multi-phase CT or MRI, vs multi-phase CT or MRI alone in detecting HCC tumors, is needed to improve the evaluation of treatment response in this disease.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: November 2, 2019
First decision: November 22, 2019
Article in press: January 14, 2020
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bramhall SR, Corrales FJ, Guo JS, Niu ZS, Sun XT, Tajiri K S-Editor: Zhang L L-Editor: A E-Editor: Qi LL
Contributor Information
Jason T Cheng, Transplantation Institute, Loma Linda University Medical Center, San Bernardino, CA 92408, United States. jtcheng@llu.edu.
Nelly E Tan, Department of Radiology, Loma Linda University Medical Center, Loma Linda, CA 92354, United States.
Michael L Volk, Transplantation Institute, Loma Linda University Medical Center, San Bernardino, CA 92408, United States.
References
- 1.World Health Organization. Cancer Fact Sheets; 2018. Available from: https://gco.iarc.fr/today/fact-sheets-cancers.
- 2.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 5.Shaw JJ, Shah SA. Rising incidence and demographics of hepatocellular carcinoma in the USA: what does it mean? Expert Rev Gastroenterol Hepatol. 2011;5:365–370. doi: 10.1586/egh.11.20. [DOI] [PubMed] [Google Scholar]
- 6.Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, Massironi S, Della Corte C, Ronchi G, Rumi MG, Biondetti P, Colombo M. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–644. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530–547. doi: 10.1102/1470-7330.2012.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792–797. doi: 10.1016/s0168-8278(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 10.Wolfort RM, Papillion PW, Turnage RH, Lillien DL, Ramaswamy MR, Zibari GB. Role of FDG-PET in the evaluation and staging of hepatocellular carcinoma with comparison of tumor size, AFP level, and histologic grade. Int Surg. 2010;95:67–75. [PubMed] [Google Scholar]
- 11.Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, Brown JJ, McFarland EG, Middleton WD, Balfe DM, Ritter JH. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226:533–542. doi: 10.1148/radiol.2262011980. [DOI] [PubMed] [Google Scholar]
- 12.Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, Lu DS. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161–167. doi: 10.1016/j.cgh.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 14.Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401–421. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 15.Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213–221. [PubMed] [Google Scholar]
- 16.Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med. 2007;48:902–909. doi: 10.2967/jnumed.106.036673. [DOI] [PubMed] [Google Scholar]
- 17.Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, Lee WJ, Kim CM, Nam BH. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912–1921. doi: 10.2967/jnumed.108.055087. [DOI] [PubMed] [Google Scholar]
- 18.Hwang KH, Choi DJ, Lee SY, Lee MK, Choe W. Evaluation of patients with hepatocellular carcinomas using [(11) C] acetate and [(18) F] FDG PET/CT: A preliminary study. Appl Radiat Isot. 2009;67:1195–1198. doi: 10.1016/j.apradiso.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, Fung JY, Yan Chan AC, Sharr W, Yau T, Poon RT, Fan ST. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon's perspective. J Nucl Med. 2013;54:192–200. doi: 10.2967/jnumed.112.107516. [DOI] [PubMed] [Google Scholar]