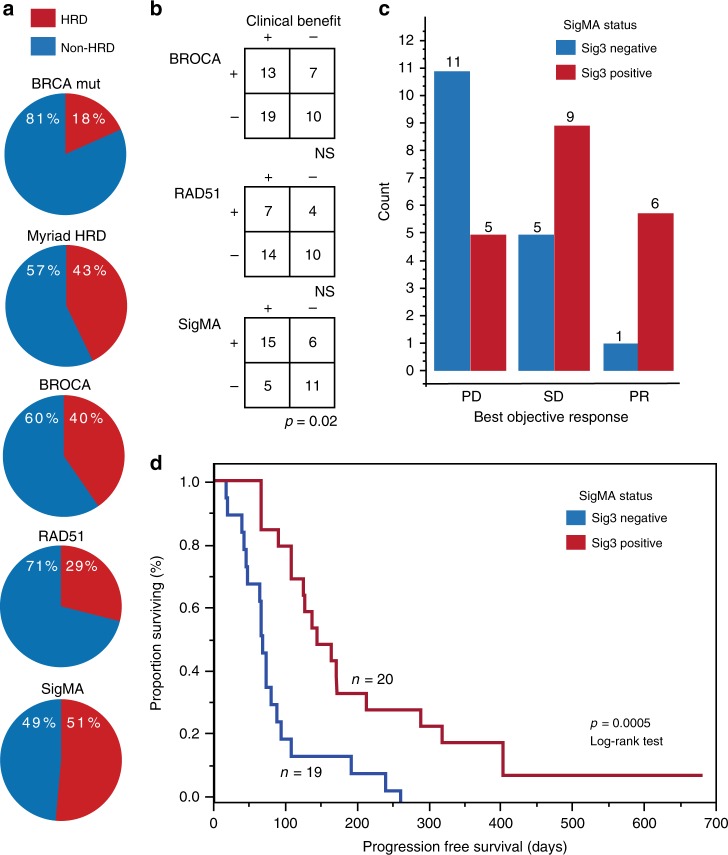
Fig. 1. Tumor mutational signature 3 positivity associates with prolonged progression-free survival with the combination of niraparib and pembrolizumab.
a SigMA identified a larger proportion of tumors positive for homologous recombination deficiency (HRD). The proportions of tumors positive (red) and negative (blue) for HRD as annotated by the BRCA1/2 mutation, Myriad HRD test, BROCA, RAD51, and SigMA. b Sig3 positivity is associated with clinical benefit as determined by either complete or partial response or stable disease. Correlations of HRD to clinical benefit (Fisher’s exact test). c Proportions of patients positive (red) or negative (blue) for Sig3 according to best objective response. PD progressive disease, SD Stable disease, PR partial response. d Sig3 associates with increased progression-free survival (PFS; Log-rank test). Kaplan–Meier graph for PFS for the combination of niraparib and pembrolizumab according to Sig3 status. All test were two-sided.