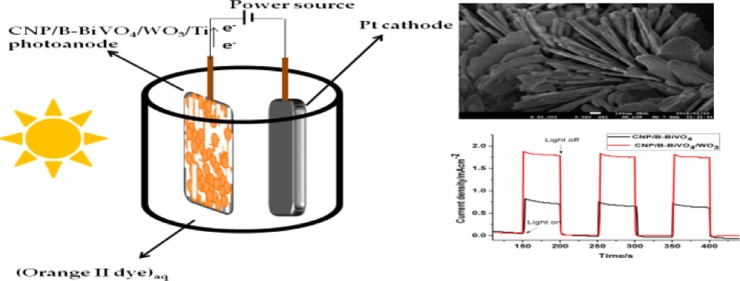
Abstract
We report the preparation and application of a heterostructured photoelectrocatalyst comprising carbon nanoparticles (CNPs) and boron codoped BiVO4 and WO3 for the removal of an organic dye pollutant in water. The materials, synthesized by hydrothermal method, were characterized by X-ray diffraction, diffuse reflectance UV–visible spectroscopy, energy-dispersive X-ray spectroscopy, and electron microscopy. The catalysts were immobilized on treated titanium sheets by drop-casting. The fabricated electrodes were characterized by linear sweep voltammetry (LSV) and chronoamperometry. Diffuse reflectance spectroscopy of the catalysts reveals that the incorporation of CNPs and B into the structure of monoclinic BiVO4 enhanced its optical absorption in both UV and visible regions. The LSV measurements carried out in 0.1 M Na2SO4 showed that the BiVO4- and WO3-based photoelectrode demonstrated significant photoactivity. CNP/B-BiVO4 and CNP/B-BiVO4/WO3 photoanodes gave photocurrent densities of approximately 0.83 and 1.79 mA/cm2, respectively, at 1.2 V (vs 3 M Ag/AgCl). The performance of the electrodes toward degradation of orange II dye was in the order BiVO4 < B-BiVO4 < WO3 < CNP-BiVO4 < CNP/B-BiVO4 < CNP/B-BiVO4/WO3, and the apparent rate constants obtained by fitting the experimental data into the Langmuir Hinshelwood kinetic model are 0.0924, 0.1812, 0.254, and 0.845 h–1 for BiVO4, WO3, CNP/B-BiVO4, and CNP/B-BiVO4/WO3, respectively. The chemical oxygen demand abatement after 3 h of electrolysis at the best performing photoanode was 58%. The study showed that BiVO4 and WO3 are promising anodic materials for photoelectrocatalytic water treatment plant.
1. Introduction
Photoelectrocatalytic (PEC) process has been indicated to hold promise for wastewater remediation and energy conversion.1 In this technique, electrical energy is applied to a photocatalytic system to obtain synergistic benefit. In the last few years, combining electrochemical process and photocatalysis has been shown to yield improved performance for organic pollutant degradation compared to either electro-oxidation or photocatalysis alone. This enhanced performance has been attributed to the ability of the applied bias potential to promote the separation of the photogenerated hole and electron pairs.2−4
Photocatalysts that are sensitive in the visible region of the solar spectrum have received considerable attention as anodic materials for PEC5−7 because utilizing sunlight for the process promotes sustainability. For instance, BiVO4 and its composites have been used as photoanodes in the PEC system for hydrogen production via water splitting and organic contaminant decomposition.8−12 Having a narrow band gap of 2.4 eV, BiVO4 is an ideal semiconductor for visible-light-driven processes. It is nontoxic, stable to photocorrosion, inexpensive, and can be obtained via simple synthetic routes.13 However, apart from the rapid recombination of electron–hole pairs,14 BiVO4 also suffers from poor electrical conductivity and low adsorptive performance.15 These drawbacks make BiVO4 an inefficient photocatalyst for pollutant degradation. Studies have shown that the photocatalytic/PEC degradation of organic pollutants at pristine BiVO4 presented low removal efficiency.16−19
WO3 (band gap, 2.5–2.8 eV) is another promising material for PEC. It is inexpensive, demonstrates low susceptibility to photocorrosion, exhibits appreciable stability in acidic and oxidative conditions, and nontoxic.20−22 Importantly, WO3 is being explored as a potential photocatalyst for destruction of organic pollutants because of the high oxidation power of its valence-bound holes.23 In spite of its impressive properties, the photocatalytic activity of WO3 is poor owing to its relatively low conduction band edge. The position of the conduction band edge is unfavorable for one-electron reduction of adsorbed oxygen molecules.24 This leads to accumulation of photogenerated electrons and their consequent recombination with the holes which are needed for oxidation of target compounds. As a result, improving the photocatalytic activity of WO3 by modifying its electronic structure has been the focus of many studies. It has been reported that tuning the morphology/structure of WO3 can yield a catalyst with enhanced charge separation and improved photoactivity.20,25−28 Theerthagiri et al., Zhang et al., and Yao et al., in separate studies, showed that WO3 nanorods possess suitable physicochemical properties for enhanced photocatalytic performance.29−31
Fabricating a hybrid of two or more semiconductors has been hinted as one of the plausible approaches to overcome the challenges of electron and hole recombination.32−36 Similarly, some other limitations associated with individual photocatalyst such as the low conduction band edge in WO3 and the poor charge mobility in BiVO4 can also be circumvented by this approach.3 The resulting nanocomposite catalysts have been reported to display much higher activity than the individual materials. Xia et al. reported the oxidation of phenol on a Fe2O3/BiVO4 thin film anode via PEC process.37 The composite photoanode demonstrated superior performance to the pristine BiVO4 anode. The authors attributed the enhanced degradation performance to the ultrathin iron oxide, which minimized charge carrier recombination in BiVO4. In another report by Martins et al.,38 WO3-modified TiO2 nanotube array (NTA) exhibited better mineralization efficiency than the unmodified TiO2 (NTAs) when both electrodes were employed for the PEC degradation of the endocrine-disrupting compound propyl paraben. Similarly, Wei et al.39 reported that decontamination of coking wastewater containing phenolic compounds was much more efficient at heterostructured TiO2/g-C3N4 than at pure TiO2 and g-C3N4. The improvement in the PEC efficiency was attributed partly to the heterojunction that is formed between the two photocatalysts which suppresses the recombination rate of electrons and holes.
In this work, BiVO4 has been modified with carbon nanoparticles (CNPs) and boron to obtain a photocatalyst denoted as CNP/B-BiVO4. This catalyst was then coupled to WO3 nanorods and the resulting nanocomposite was immobilized on Ti plate. The photoanode, CNP/B-BiVO4/WO3, was then employed for the catalytic oxidation of orange II dye. A few studies have reported the use of BiVO4/WO3 as anodic material in PEC experiments.32,40,41 In this study, we hypothesized that modification of BiVO4 with substances such as CNP and B would impact positively on the photocatalytic performance of the semiconductor. In addition, we envisaged that coupling this modified material to WO3 nanorods will provide a nanocomposite anode with desirable properties for PEC applications.
2. Results and Discussion
2.1. XRD Analyses
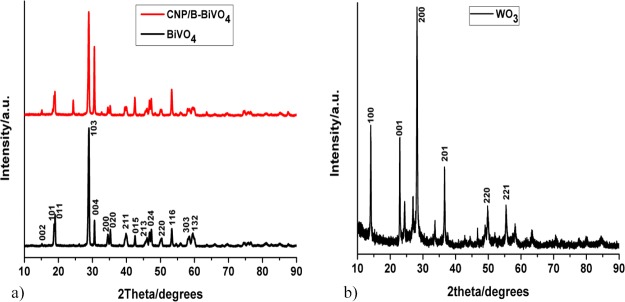
The X-ray diffraction (XRD) patterns of the electrode materials are shown in Figure 1. The diffraction peaks in the XRD pattern of pure BiVO4 (Figure 1) can be indexed to monoclinic scheelite structure of BiVO4 (JCPDS card no. 14-0688).42,43 The XRD pattern of CNPs and B codoped BiVO4 shows an additional peak at 24°, which can be ascribed to the (002) plane of graphite.
Figure 1.
Powder XRD patterns of (a) BiVO4 and CNP/B-BiVO4 and (b) WO3.
The XRD pattern of WO3 (Figure 1b) shows diffraction peaks at 2θ = 14.1, 22.9, 28.2, 36.5, 49.9, and 55.4°, which correspond to the (100), (001), (200), (201), (220), and (221) crystal planes, respectively, of the hexagonal structure of WO3 (JCPDS card no. 75-2187).44,45
2.2. Morphology Investigations, EDS Analysis, and Elemental Mapping
The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images, energy-dispersive spectroscopy (EDS) spectra, and elemental maps are presented in Figures 2 and 3. From the SEM image of pristine BiVO4 (Figure 2a), it can be observed that the sizes of the nanoparticles formed are largely uniform. The particles seem to be closely packed. The SEM image of the doped BiVO4 (Figure 2b) shows a morphological characteristic of a composite material. It shows some plate-like structures with embedded particles and the constituents seem to be well integrated. The electron images show that the dimensions of the materials are much less than 100 nm. The elemental map (Figure 2c) confirms the presence of B in the nanocomposite, with the element uniformly distributed within the material. Similarly, Figure 2d reveals that carbon particles are present in the nanocomposite material. The EDS spectrum (Figure 2e) further confirms the presence of B, although it has a very low intensity owing to its very low concentration compared with the other elements in the composite material.
Figure 2.
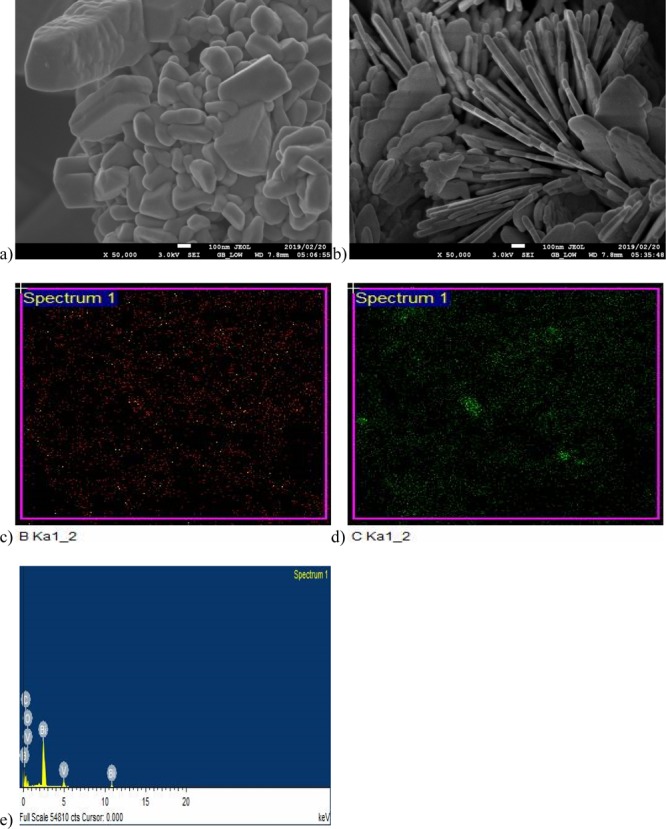
SEM images of (a) BiVO4 and (b) CNP/B-BiVO4, and elemental maps for (c) B and (d) C. (e) EDS spectrum of CNP/B-BiVO4.
Figure 3.
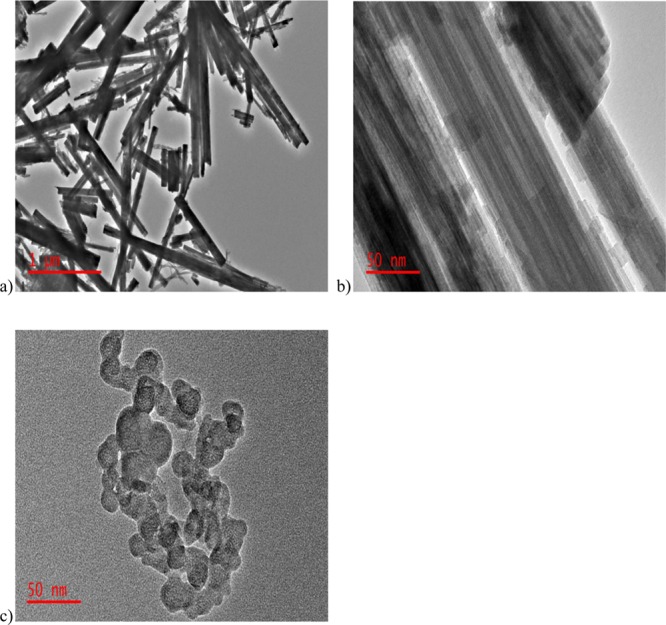
TEM images of (a,b) WO3 and (c) CNP.
The TEM images of the synthesized WO3 nanorods are shown in Figure 3a.b. The image at 1 μm (Figure 3a) reveals that the rods are of different lengths, but the diameter seems to be essentially the same. The TEM image of the carbon particles is presented in Figure 3c, where the particles appear to be spherical and agglomerated.
2.3. BET Surface Area Analysis
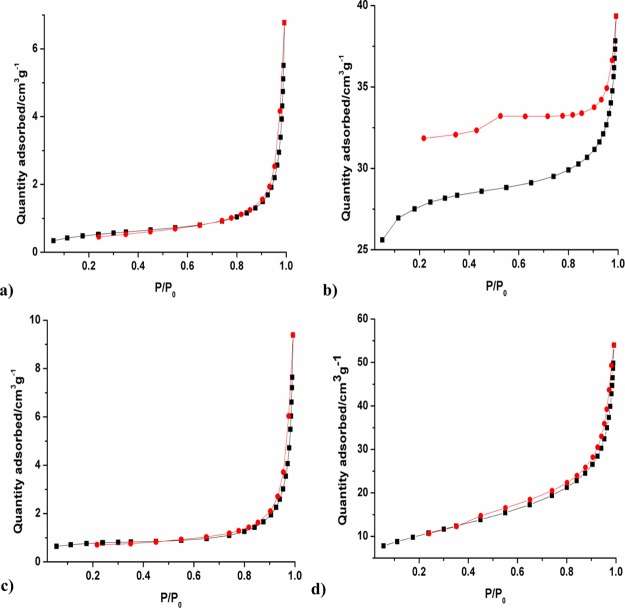
Nitrogen adsorption–desorption experiment was performed to investigate the Brunauer–Emmett–Teller (BET) surface area of the pristine and composite materials. The adsorption isotherms of BiVO4, CNP, CNP/B-BiVO4, and WO3 are given in Figure 4, and the specific BET surface areas are presented in Table 1. From the values in the table, it is obvious that incorporation of CNP into the lattices of BiVO4 resulted in an increase in the surface area of the photocatalyst. Higher surface area is beneficial for interaction between the organic pollutant and the photoelectrocatalyst, and this will aid overall degradation efficiency. Similarly, the comparatively high BET surface area of WO3 nanorods will contribute significantly to the adsorption capability of the heterojunction photoanode, which will enhance its catalytic performance.
Figure 4.
Nitrogen adsorption–desorption isotherms of (a) pristine BiVO4, (b) CNP, (c) CNP/B-BiVO4, and (d) WO3.
Table 1. BET Surface Area of Pristine BiVO4, CNP, Doped BiVO4, and WO3.
| sample | BET surface area/m2 g–1 |
|---|---|
| BiVO4 | 1.81 |
| CNP | 84.32 |
| CNP/B-BiVO4 | 2.49 |
| WO3 | 36.26 |
2.4. Diffuse Reflectance Spectroscopy
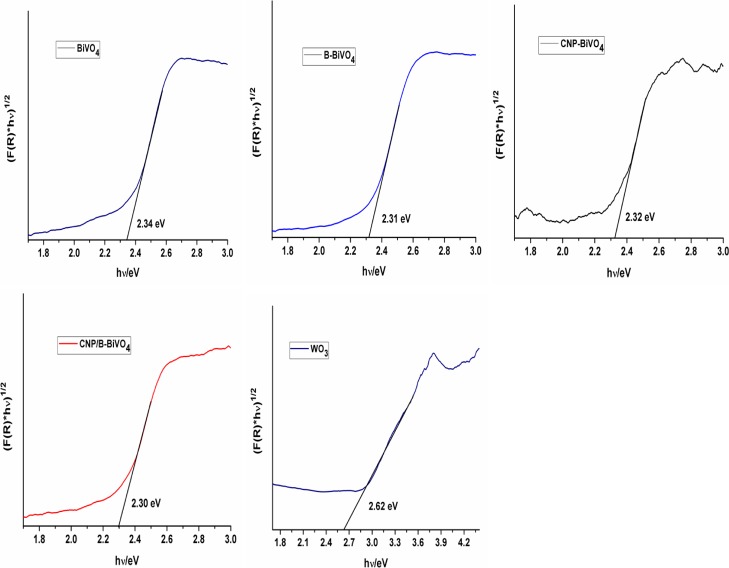
Diffuse reflectance spectroscopy (DRS) is a valuable technique that can be used to gain insights into the electronic states of semiconducting materials. The UV–vis absorption spectra of BiVO4, WO3, and CNP/B-BiVO4 are shown in Figure 5. The intrinsic absorption bands of both the pristine BiVO4 and doped BiVO4 span over the UV and visible light regions, while that of WO3 seem to be strong within the UV region, extending slightly into the visible region. This absorption characteristic of WO3 is in agreement with earlier reports.46,47 The doped BiVO4 demonstrates superior visible light absorption compared to the pristine BiVO4. In addition, the absorption edge of the CNP and B codoped BiVO4 exhibits a red shift. The extension of the absorption edge of the doped material may be as a result of band transitions occurring because of the presence of impurities in the forbidden band.48 The band gap energies of the catalysts (Figure 6) were estimated from a plot of (F(R) × hν)1/2 against hν, where F(R) is the Kubelka–Munk function given by (1 – R)2/2 × R (R is reflectance), and hν is the incident photon energy.49,50
Figure 5.
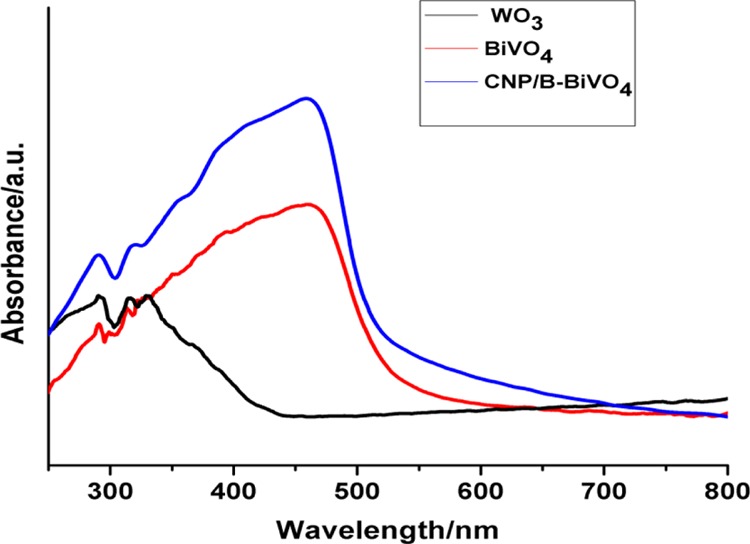
UV–vis absorption spectra of BiVO4, WO3 and CNP/B-BiVO4.
Figure 6.
Band gap energies of the catalysts estimated from the plot of Kubelka–Munk function vs incident energy.
2.5. Linear Sweep Voltammetry and Photocurrent Response
The linear sweep voltammograms of the fabricated photoanodes were obtained in the dark and in the presence of light. As shown in Figure 7a, the current responses of both CNP/B-BiVO4 and CNP/B-BiVO4/WO3 in the dark are not significantly different until reaching a potential of about 1.0 V, with the CNP/B-BiVO4/WO3 electrode displaying a slightly higher current than the codoped BiVO4 electrode (0.16 and 0.15 mA/cm2 at 1.3 V). Apparently, the presence of WO3 contributed to the current signal of the CNP/B-BiVO4/WO3 electrode. It is well known that WO3 exhibits better charge-transfer property than BiVO4.51,52 In addition, in both doped BiVO4 and heterostructured electrodes, photocurrent seems to increase as the anodic potential increases. In the presence of light, the photoanodes showed markedly higher photocurrents (1.25 and 2.20 mA/cm2 at 1.3 V for CNP/B-BiVO4 and CNP/B-BiVO4/WO3, respectively) than those recorded under dark condition (Figure 6b). The significant photocurrent enhancement recorded by the coupled semiconductor anode can be attributed to the contributions from both BiVO4 and WO3 (Figure 7b). The excellent charge transport property of WO3 and the high visible light activity of BiVO4 are beneficial for improved photoactivity. As evident in Figure 7b, the photocurrent responses at both CNP/B-BiVO4 and CNP/B-BiVO4/WO3 are stable over the time range shown.
Figure 7.
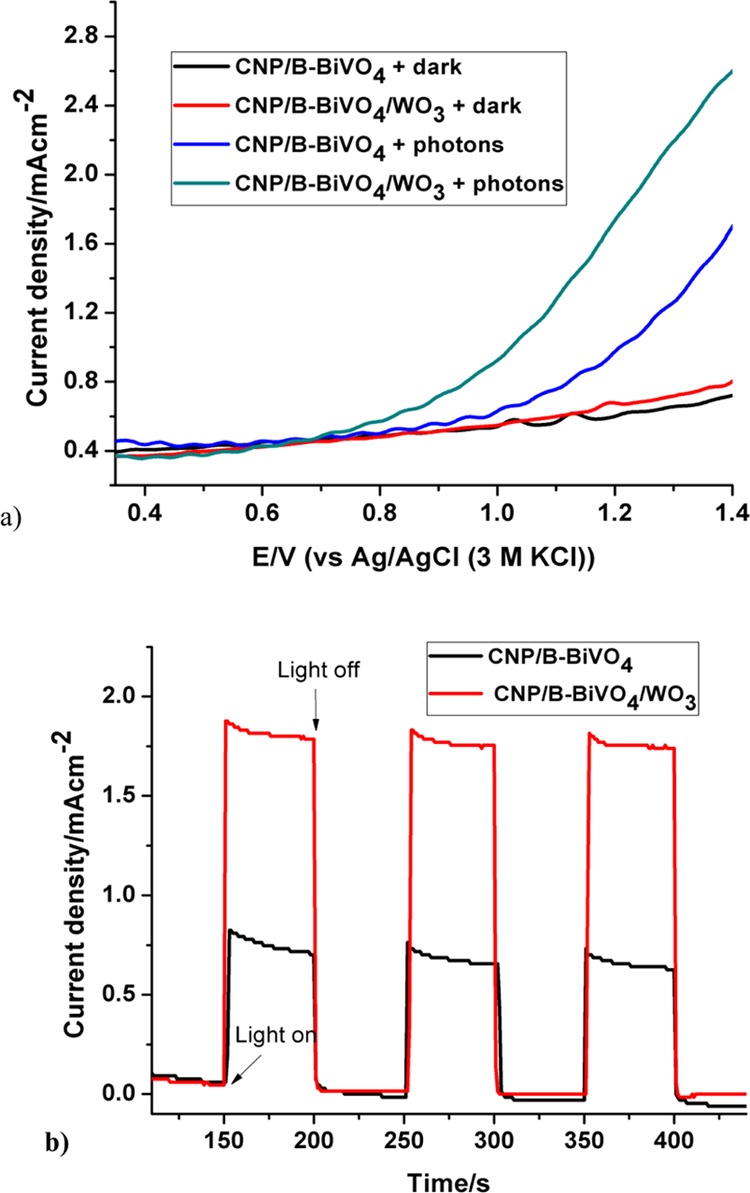
(a) Linear sweep voltammograms of photoanodes measured in 0.1 M Na2SO4 and (b) photocurrent response of CNP/B-BiVO4 and CNP/B-BiVO4/WO3 obtained in 5 mg L–1 of orange II dye (prepared in 0.1 M Na2SO4) at a potential of 1.2 V.
2.6. PEC Degradation Experiments
Degradation of orange II sodium salt was carried out at the fabricated electrodes to evaluate their PEC performance. The oxidation process was performed at an applied anodic potential of 2.0 V. The removal efficiency of the dye was calculated from the following relation:
where A0 and At are the absorbance values at time t = 0 and t = t, respectively.
As can be seen in Figure 8a, the CNP/B-BiVO4/WO3 electrode exhibited the highest degradation efficiency with 92% removal of the dye in 3 h of the PEC process. The performance of the photoanodes follows the order BiVO4 < B-BiVO4 < WO3 < CNP–BiVO4 < CNP/B-BiVO4 < CNP/B-BiVO4/WO3. Degradation efficiency at B-doped BiVO4 photoanode is only slightly higher than that obtained at the pristine BiVO4 anode (data not shown). Wang et al. made similar observation in the photocatalytic performance of BiVO4 and B-BiVO4.53 The authors reported that boron doping narrows the band gap energy of the photocatalyst by a magnitude of 0.04 eV, thus increasing its light absorption in the visible region. It was also suggested that interstitial boron doping can increase the number of oxygen vacancies and V4+, leading to red shifting of the absorption edge and consequently improved photocatalytic performance in the visible light region. It is noteworthy that the performance of pristine BiVO4 is inferior to that of the pure WO3 nanorods despite the former being a better absorber of visible light. The poor conductivity and low adsorption capability of BiVO4 are some of the factors responsible for its low PEC performance. On the other hand, the relatively large surface area of WO3 and its excellent conductivity are favorable for the degradation of organic pollutants. However, incorporation of CNP into BiVO4 leads to significant improvement in its PEC performance. In essence, the CNP and B codoped BiVO4 showed much more enhanced performance. Also, incorporation of CNP into the structure of BiVO4 could lead to improved charge transport property and enhanced adsorption capability of the photocatalyst. Apparently, the CNP/B-BiVO4/WO3 performed best because of the contributions of the various constituent materials. The kinetic rate constants obtained by fitting the degradation experimental data into the Langmuir Hinshelwood kinetic model are 0.0924, 0.1812, 0.254, and 0.845 h–1 for BiVO4, WO3, CNP/B-BiVO4, and CNP/B-BiVO4/WO3, respectively. Figure 8b displays the plots of normalized concentration abatement of the dye via photolysis, electrolysis, and photoelectrocatalysis. It can be seen that the coupled process offers a far more efficient removal of the dye than the individual processes. This observation further affirms the superiority of PEC oxidation process to both electrochemical oxidation and photocatalytic oxidation processes. The chemical oxygen demand (COD) decay after 3 h of the PEC process at the preferred anode was 58%. For possible practical application, the photoelectrocatalyst (CNP/B-BiVO4/WO3) was further employed for the degradation of 50 mg L–1 (50 mL) of the dye solution under the same experimental conditions. A 56% decolourization was obtained after 3 h of the process.
Figure 8.
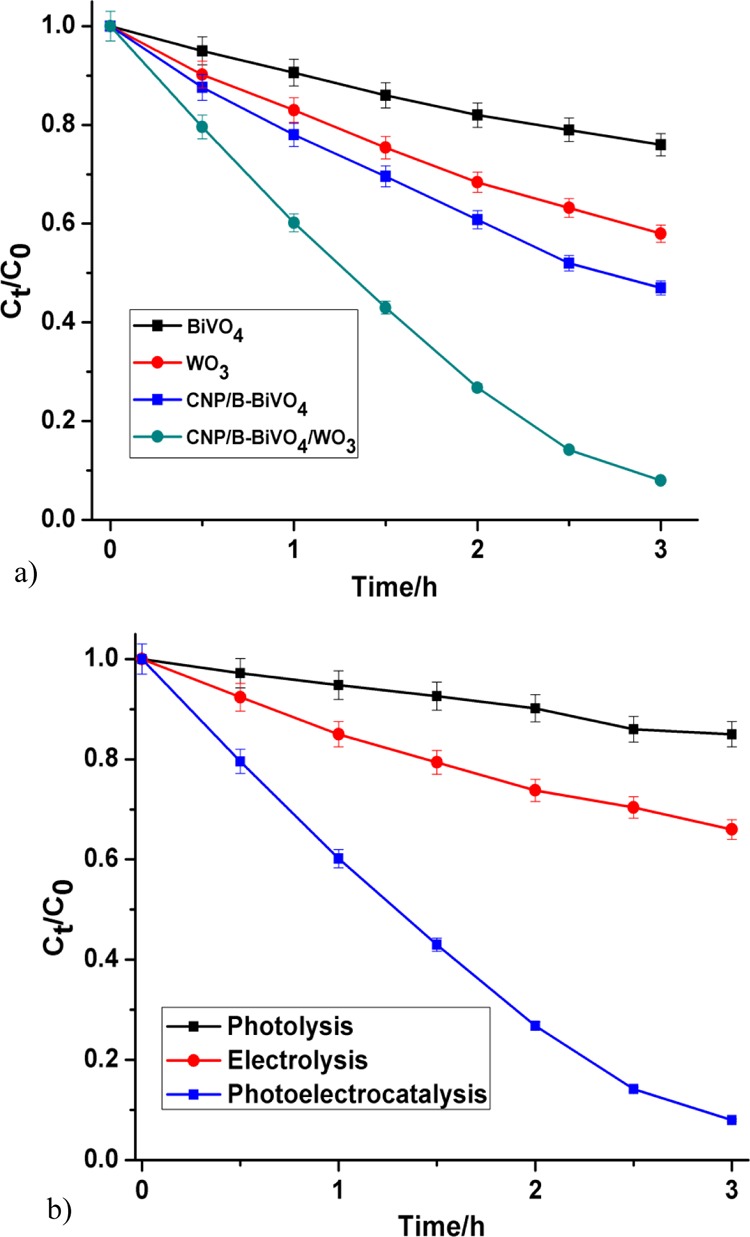
Degradation profiles of orange II sodium salt (a) at different photoanodes (b) by different oxidation processes.
3. Conclusions
Two visible-light-sensitive semiconductors, namely, BiVO4 and WO3, have been synthesized, characterized, and applied for the degradation of a model organic pollutant, orange II dye. BiVO4 was modified with CNP and B to improve its charge transport property and adsorption behavior to increase its PEC efficiency. The kinetic rate constants calculated for the degradation of the contaminants at BiVO4 and WO3 photoanodes were 0.0924 and 0.1812 h–1, respectively. The much better performance of WO3 nanorods compared to the undoped BiVO4 may not be unrelated to its morphology, which can retard recombination of photogenerated electron–hole pairs. Doping CNP and B into the structure of BiVO4 led to significant improvement in its PEC performance. Coupling the doped BiVO4 to WO3 yielded a heterostructured anodic material which showed much more excellent properties than the individual components. The higher performance of this electrode can be linked to the formation of n–n heterojunction, which promotes separation of charges and generation of a large amount of the valence-bound holes, which are needed for the oxidation of the pollutant. This study shows that BiVO4- and WO3-based anodes have prospects for water remediation via photoelectrocatalysis.
4. Experimental Section
4.1. Materials
Sodium tungstate dihydrate (Na2WO4·2H2O), bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), sodium metavanadate (NaVO3), sodium chloride (NaCl), sodium sulfate (Na2SO4), sodium hydrogen carbonate (NaHCO3), sodium hydroxide (NaOH), orange II sodium salt, polyethylene glycol (PEG), boric acid (H3BO3), and ethanol were purchased from Sigma-Aldrich, South Africa. All solutions were prepared with deionized water.
4.2. Synthesis of WO3 Nanorods
The synthesis of WO3 nanorods is as follows: 2.62 g of Na2WO4·2H2O was dissolved in 80 mL of deionized water, followed by addition of 1.0 g of NaCl. The homogeneous mixture obtained was then acidified with HCl until the pH was 2.06. Upon addition of HCl, the mixture turned to a white precipitate, and this precursor solution was transferred to a 100 mL Teflon-lined autoclave. The autoclave was sealed and maintained at 180 °C in an oven for 24 h. After cooling to room temperature, the synthesized material was transferred into centrifuge tubes and washed by centrifugation repeatedly with deionized water and then ethanol. The material was then dried at 80 °C in an air oven for 8 h. The powder material obtained after drying was kept for use.
4.3. Preparation of CNPs, BiVO4, and CNP/B-BiVO4
CNPs were prepared from oats purchased from a local market in Johannesburg, South Africa. Oats (5 g) was powdered by grinding. The powder was then put into a crucible and transferred into a muffle furnace maintained at 400 °C for 2 h. The black material formed was allowed to cool to room temperature, pulverized, and subsequently dispersed in deionized water. The dispersion was centrifuged and the supernatant was decanted into a clean beaker. This process was repeated several times to obtain more supernatants. The water in the supernatant was evaporated and the solid particles left in the beaker were collected and kept for use.
To synthesize pristine BiVO4, 0.61 g of NaVO3 was dissolved in about 40 mL of deionized water and 2.43 g of Bi(NO3)3·5H2O were dissolved in 0.1 M HNO3. The NaVO3 solution was then gradually added to the Bi(NO3)3 solution. The yellow precursor solution formed was transferred into a Teflon-lined autoclave, sealed, and put into the stainless steel shell. This was then maintained 180 °C for 24 h in an oven. Upon cooling, the material was transferred into centrifuge tubes and washed a few times with water. The yellow substance obtained after decantation was dried in an air oven at 80 °C for 8 h. Preparation of CNP/B-BiVO4 followed the same procedure except that 1.0 g of H3BO3 and 10 mg of CNP were added to the solution of NaVO3 prior to mixing with Bi(NO3)3·5H2O solution.
4.4. Characterization of the Materials
Powder XRD analysis of the catalysts was done on a Rigaku Smartlab X-ray diffractometer (USA) equipped with Cu kα radiation and operated at 40 kV and 40 mA. The electron images were obtained on a scanning electron microscope (TESCAN, VEGA3 XMU, Czech Republic) and transmission electron microscope (JEOL 2100 HRTEM 200V, Japan). Energy-dispersive X-ray spectrometry analysis was performed on the equipment attached to the scanning electron microscope. DRS was done on a UV–visible spectrophotometer (Shimadzu 2450, Japan), and concentration abatement of the dye was monitored on a UV–visible spectrophotometer (Agilent Cary 60, Malaysia) at its wavelength of maximum absorption. COD was determined on a HACH DR3900 spectrophotometer.
4.5. Fabrication of Electrodes
Ti sheets were first degreased in 40% m/m NaOH solution heated to 80 °C and maintained for 2 h. Etching of the sheets was then carried out in a 18% v/v HCl solution maintained at 98% for 2 h.
The dispersions of BiVO4, B-BiVO4, CNP/BiVO4, CNP/B-BiVO4, and WO3 were prepared by weighing 0.2 g of each of the catalysts into an ethanolic solution of PEG (containing 2 mL of ethanol and 1 mL of PEG). A mixture of both CNP/B-BiVO4 and WO3 was also prepared by dispersing 0.1 g of each of the catalysts in an ethanolic solution of PEG. The dispersions were ultrasonicated for 60 min.
The dispersion (100 μL) was drop-cast onto both sides of the prepared Ti sheets with an exposed area of 4 cm2. The coated sheets were dried in an air oven at a temperature of 250 °C. The coating was repeated once more and the sheets were sintered at 500 °C in a muffle furnace.
4.6. Electrochemical Experiments
Electrochemical measurements were done on a computer-controlled potentiostat (Autolab PGSTAT 302N) using a three-electrode system. The working electrodes used include BiVO4/Ti, B-BiVO4/Ti, CNP–BiVO4/Ti, CNP/B-BiVO4/Ti, WO3/Ti, and CNP/B-BiVO4/WO3/Ti, the reference electrode was Ag/AgCl (3 M KCl), and the counter electrode was a coiled platinum wire. Linear sweep voltammetric measurements were carried out in a 0.1 M solution of Na2SO4, and amperometric measurements in the presence and absence of photons were performed in 5 mg L–1 solution of orange II sodium salt (prepared in 0.1 M Na2SO4). Electrochemical oxidation of the dye was carried out in a quartz photoelectrochemical cell in the presence and absence of light. Typically, 250 mL of the simulated wastewater (containing 5 mg L–1 of the analyte) was electrolyzed in an experiment with continuous stirring of the electrolytic solution. The solar light source was an Oriel solar simulator. The simulator has a xenon lamp of 100 W and a UV cutoff filter (Air Mass 1.5 Global filter), giving 1.0 sun output. The distance between the photoelectrochemical cell and the light source was about 5 cm.
Acknowledgments
Financial supports from the following institutions in South Africa are gratefully acknowledged: Faculty of Science, University of Johannesburg; DST/Mintek Nanotechnology Innovation Centre, University of Johannesburg; Centre for Nanomaterials Science Research, University of Johannesburg; National Research Foundation of South Africa (CPRR grant number: 98887); and Water Research Commission of South Africa (grant number. K5/2567).
The authors declare no competing financial interest.
References
- Zhang X.; Yang Y.; Ding S.; Que W.; Zheng Z.; Du Y. Construction of High-Quality SnO2@ MoS2 Nanohybrids for Promising Photoelectrocatalytic Applications. Inorg. Chem. 2017, 56, 3386–3393. 10.1021/acs.inorgchem.6b02914. [DOI] [PubMed] [Google Scholar]
- Peleyeju M. G.; Umukoro E. H.; Tshwenya L.; Moutloali R.; Babalola J. O.; Arotiba O. A. Photoelectrocatalytic water treatment systems: degradation, kinetics and intermediate products studies of sulfamethoxazole on a TiO2–exfoliated graphite electrode. RSC Adv. 2017, 7, 40571–40580. 10.1039/c7ra07399b. [DOI] [Google Scholar]
- Peleyeju M. G.; Arotiba O. A. Recent trend in visible-light photoelectrocatalytic systems for degradation of organic contaminants in water/wastewater. Environ. Sci.: Water Res. Technol. 2018, 4, 1389–1411. 10.1039/c8ew00276b. [DOI] [Google Scholar]
- Bessegato G. G.; Cardoso J. C.; Zanoni M. V. B. Enhanced photoelectrocatalytic degradation of an acid dye with boron-doped TiO2 nanotube anodes. Catal. Today 2015, 240, 100–106. 10.1016/j.cattod.2014.03.073. [DOI] [Google Scholar]
- Deng W.; Zhao H.; Pan F.; Feng X.; Jung B.; Abdel-Wahab A.; Batchelor B.; Li Y. Visible-light-driven photocatalytic degradation of organic water pollutants promoted by sulfite addition. Environ. Sci. Technol. 2017, 51, 13372–13379. 10.1021/acs.est.7b04206. [DOI] [PubMed] [Google Scholar]
- Rincón N. C.; Hammouda S. B.; Sillanpää M.; Barrios V. E. Enhanced photocatalytic performance of zinc oxide nanostructures via photoirradiation hybridisation with graphene oxide for the degradation of triclosan under visible light: Synthesis, characterisation and mechanistic study. J. Environ. Chem. Eng. 2018, 6, 6554–6567. 10.1016/j.jece.2018.09.064. [DOI] [Google Scholar]
- Ma R.; Zhang S.; Li L.; Gu P.; Wen T.; Khan A.; Li S.; Li B.; Wang S.; Wang X. Enhanced Visible-Light-Induced Photoactivity of Type-II CeO2/g-C3N4 Nanosheet toward Organic Pollutants Degradation. ACS Sustainable Chem. Eng. 2019, 7, 9699–9708. 10.1021/acssuschemeng.9b01477. [DOI] [Google Scholar]
- Monfort O.; Pop L.-C.; Sfaelou S.; Plecenik T.; Roch T.; Dracopoulos V.; Stathatos E.; Plesch G.; Lianos P. Photoelectrocatalytic hydrogen production by water splitting using BiVO4 photoanodes. Chem. Eng. J. 2016, 286, 91–97. 10.1016/j.cej.2015.10.043. [DOI] [Google Scholar]
- Zhou Y.; Zhang L.; Lin L.; Wygant B. R.; Liu Y.; Zhu Y.; Zheng Y.; Mullins C. B.; Zhao Y.; Zhang X.; Yu G. Highly efficient photoelectrochemical water splitting from hierarchical WO3/BiVO4 nanoporous sphere arrays. Nano Lett. 2017, 17, 8012–8017. 10.1021/acs.nanolett.7b04626. [DOI] [PubMed] [Google Scholar]
- Monfort O.; Raptis D.; Satrapinskyy L.; Roch T.; Plesch G.; Lianos P. Production of hydrogen by water splitting in a photoelectrochemical cell using a BiVO4/TiO2 layered photoanode. Electrochim. Acta 2017, 251, 244–249. 10.1016/j.electacta.2017.08.125. [DOI] [Google Scholar]
- Tolod K.; Hernández S.; Russo N. Recent advances in the BiVO4 photocatalyst for sun-driven water oxidation: top-performing photoanodes and scale-up challenges. Catalysts 2017, 7, 13. 10.3390/catal7010013. [DOI] [Google Scholar]
- Monfort O.; Sfaelou S.; Satrapinskyy L.; Plecenik T.; Roch T.; Plesch G.; Lianos P. Comparative study between pristine and Nb-modified BiVO4 films employed for photoelectrocatalytic production of H2 by water splitting and for photocatalytic degradation of organic pollutants under simulated solar light. Catal. Today 2017, 280, 51–57. 10.1016/j.cattod.2016.07.006. [DOI] [Google Scholar]
- Ahmed T.; Zhang H.-l.; Xu H.-b.; Zhang Y. m-BiVO4 hollow spheres coated on carbon fiber with superior reusability as photocatalyst. Colloids Surf., A 2017, 531, 213–220. 10.1016/j.colsurfa.2017.08.004. [DOI] [Google Scholar]
- Malathi A.; Vasanthakumar V.; Arunachalam P.; Madhavan J.; Ghanem M. A. A low cost additive-free facile synthesis of BiFeWO6/BiVO4 nanocomposite with enhanced visible-light induced photocatalytic activity. J. Colloid Interface Sci. 2017, 506, 553–563. 10.1016/j.jcis.2017.07.079. [DOI] [PubMed] [Google Scholar]
- He Z.; Shi Y.; Gao C.; Wen L.; Chen J.; Song S. BiOCl/BiVO4 p–n heterojunction with enhanced photocatalytic activity under visible-light irradiation. J. Phys. Chem. C 2014, 118, 389–398. 10.1021/jp409598s. [DOI] [Google Scholar]
- Sun Y.; Qu B.; Liu Q.; Gao S.; Yan Z.; Yan W.; Pan B.; Wei S.; Xie Y. Highly efficient visible-light-driven photocatalytic activities in synthetic ordered monoclinic BiVO4 quantum tubes–graphene nanocomposites. Nanoscale 2012, 4, 3761–3767. 10.1039/c2nr30371j. [DOI] [PubMed] [Google Scholar]
- Shang M.; Wang W.; Sun S.; Ren J.; Zhou L.; Zhang L. Efficient visible light-induced photocatalytic degradation of contaminant by spindle-like PANI/BiVO4. J. Phys. Chem. C 2009, 113, 20228–20233. 10.1021/jp9067729. [DOI] [Google Scholar]
- Geng Y.; Zhang P.; Li N.; Sun Z. Synthesis of Co doped BiVO4 with enhanced visible-light photocatalytic activities. J. Alloys Compd. 2015, 651, 744–748. 10.1016/j.jallcom.2015.08.123. [DOI] [Google Scholar]
- Sun J.; Guo Y.; Wang Y.; Cao D.; Tian S.; Xiao K.; Mao R.; Zhao X. H2O2 assisted photoelectrocatalytic degradation of diclofenac sodium at gC3N4/BiVO4 photoanode under visible light irradiation. Chem. Eng. J. 2018, 332, 312–320. 10.1016/j.cej.2017.09.041. [DOI] [Google Scholar]
- Dong P.; Hou G.; Xi X.; Shao R.; Dong F. WO 3-based photocatalysts: morphology control, activity enhancement and multifunctional applications. Environ. Sci.: Nano 2017, 4, 539–557. 10.1039/c6en00478d. [DOI] [Google Scholar]
- Sfaelou S.; Pop L.-C.; Monfort O.; Dracopoulos V.; Lianos P. Mesoporous WO3 photoanodes for hydrogen production by water splitting and PhotoFuelCell operation. Int. J. Hydrogen Energy 2016, 41, 5902–5907. 10.1016/j.ijhydene.2016.02.063. [DOI] [Google Scholar]
- Hilliard S.; Baldinozzi G.; Friedrich D.; Kressman S.; Strub H.; Artero V.; Laberty-Robert C. Mesoporous thin film WO3 photoanode for photoelectrochemical water splitting: a sol–gel dip coating approach. Sustainable Energy Fuels 2017, 1, 145–153. 10.1039/c6se00001k. [DOI] [Google Scholar]
- Weng B.; Wu J.; Zhang N.; Xu Y.-J. Observing the role of graphene in boosting the two-electron reduction of oxygen in graphene–WO3 nanorod photocatalysts. Langmuir 2014, 30, 5574–5584. 10.1021/la4048566. [DOI] [PubMed] [Google Scholar]
- Sakai Y.; Shimanaka A.; Shioi M.; Kato S.; Satokawa S.; Kojima T.; Yamasaki A. Fabrication of high-sensitivity palladium loaded tungsten trioxide photocatalyst by photodeposite method. Catal. Today 2015, 241, 2–7. 10.1016/j.cattod.2014.07.044. [DOI] [Google Scholar]
- Huang Z.-F.; Song J.; Pan L.; Zhang X.; Wang L.; Zou J.-J. Tungsten oxides for photocatalysis, electrochemistry, and phototherapy. Adv. Mater. 2015, 27, 5309–5327. 10.1002/adma.201501217. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Su J.; Guo L. Morphology engineering of WO 3/BiVO 4 heterojunctions for efficient photocatalytic water oxidation. CrystEngComm 2016, 18, 8961–8970. 10.1039/c6ce01952h. [DOI] [Google Scholar]
- Dirany N.; Arab M.; Leroux C.; Villain S.; Madigou V.; Gavarri J. R. Effect of WO3 nanoparticles morphology on the catalytic properties. Mater. Today: Proc. 2016, 3, 230–234. 10.1016/j.matpr.2016.01.062. [DOI] [Google Scholar]
- Ahmed B.; Kumar S.; Ojha A. K.; Donfack P.; Materny A. Facile and controlled synthesis of aligned WO3 nanorods and nanosheets as an efficient photocatalyst material. Spectrochim. Acta, Part A 2017, 175, 250–261. 10.1016/j.saa.2016.11.044. [DOI] [PubMed] [Google Scholar]
- Theerthagiri J.; Senthil R. A.; Malathi A.; Selvi A.; Madhavan J.; Ashokkumar M. Synthesis and characterization of a CuS–WO 3 composite photocatalyst for enhanced visible light photocatalytic activity. RSC Adv. 2015, 5, 52718–52725. 10.1039/c5ra06512g. [DOI] [Google Scholar]
- Zhang J.; Ma Y.; Du Y.; Jiang H.; Zhou D.; Dong S. Carbon nanodots/WO3 nanorods Z-scheme composites: Remarkably enhanced photocatalytic performance under broad spectrum. Appl. Catal., B 2017, 209, 253–264. 10.1016/j.apcatb.2017.03.017. [DOI] [Google Scholar]
- Yao S.; Qu F.; Wang G.; Wu X. Facile hydrothermal synthesis of WO3 nanorods for photocatalysts and supercapacitors. J. Alloys Compd. 2017, 724, 695–702. 10.1016/j.jallcom.2017.07.123. [DOI] [Google Scholar]
- Xia L.; Bai J.; Li J.; Zeng Q.; Li X.; Zhou B. A highly efficient BiVO4/WO3/W heterojunction photoanode for visible-light responsive dual photoelectrode photocatalytic fuel cell. Appl. Catal., B 2016, 183, 224–230. 10.1016/j.apcatb.2015.10.050. [DOI] [Google Scholar]
- Ho-Kimura S.; Moniz S. J. A.; Handoko A. D.; Tang J. Enhanced photoelectrochemical water splitting by nanostructured BiVO4–TiO2 composite electrodes. J. Mater. Chem. A 2014, 2, 3948–3953. 10.1039/c3ta15268e. [DOI] [Google Scholar]
- Kim E. S.; Kang H. J.; Magesh G.; Kim J. Y.; Jang J.-W.; Lee J. S. Improved photoelectrochemical activity of CaFe2O4/BiVO4 heterojunction photoanode by reduced surface recombination in solar water oxidation. ACS Appl. Mater. Interfaces 2014, 6, 17762–17769. 10.1021/am504283t. [DOI] [PubMed] [Google Scholar]
- Wang S.; Yun J.-H.; Luo B.; Butburee T.; Peerakiatkhajohn P.; Thaweesak S.; Xiao M.; Wang L. Recent progress on visible light responsive heterojunctions for photocatalytic applications. J. Mater. Sci. Technol. 2017, 33, 1–22. 10.1016/j.jmst.2016.11.017. [DOI] [Google Scholar]
- Yuan Y.-J.; Wang F.; Hu B.; Lu H.-W.; Yu Z.-T.; Zou Z.-G. Significant enhancement in photocatalytic hydrogen evolution from water using a MoS 2 nanosheet-coated ZnO heterostructure photocatalyst. Dalton Trans. 2015, 44, 10997–11003. 10.1039/c5dt00906e. [DOI] [PubMed] [Google Scholar]
- Xia L.; Bai J.; Li J.; Zeng Q.; Li L.; Zhou B. High-performance BiVO4 photoanodes cocatalyzed with an ultrathin α-Fe2O3 layer for photoelectrochemical application. Appl. Catal., B 2017, 204, 127–133. 10.1016/j.apcatb.2016.11.015. [DOI] [Google Scholar]
- Martins A. S.; Nuñez L.; Lanza M. R. d. V. Enhanced photoelectrocatalytic performance of TiO2 nanotube array modified with WO3 applied to the degradation of the endocrine disruptor propyl paraben. J. Electroanal. Chem. 2017, 802, 33–39. 10.1016/j.jelechem.2017.08.040. [DOI] [Google Scholar]
- Wei Z.; Liang F.; Liu Y.; Luo W.; Wang J.; Yao W.; Zhu Y. Photoelectrocatalytic degradation of phenol-containing wastewater by TiO2/gC3N4 hybrid heterostructure thin film. Appl. Catal., B 2017, 201, 600–606. 10.1016/j.apcatb.2016.09.003. [DOI] [Google Scholar]
- Zeng Q.; Li J.; Li L.; Bai J.; Xia L.; Zhou B. Synthesis of WO3/BiVO4 photoanode using a reaction of bismuth nitrate with peroxovanadate on WO3 film for efficient photoelectrocatalytic water splitting and organic pollutant degradation. Appl. Catal., B 2017, 217, 21–29. 10.1016/j.apcatb.2017.05.072. [DOI] [Google Scholar]
- Chatchai P.; Nosaka A. Y.; Nosaka Y. Photoelectrocatalytic performance of WO3/BiVO4 toward the dye degradation. Electrochim. Acta 2013, 94, 314–319. 10.1016/j.electacta.2013.01.152. [DOI] [Google Scholar]
- Liu Y.; Huang B.; Dai Y.; Zhang X.; Qin X.; Jiang M.; Whangbo M.-H. Selective ethanol formation from photocatalytic reduction of carbon dioxide in water with BiVO4 photocatalyst. Catal. Commun. 2009, 11, 210–213. 10.1016/j.catcom.2009.10.010. [DOI] [Google Scholar]
- Yan Y.; Sun S.; Song Y.; Yan X.; Guan W.; Liu X.; Shi W. Microwave-assisted in situ synthesis of reduced graphene oxide-BiVO4 composite photocatalysts and their enhanced photocatalytic performance for the degradation of ciprofloxacin. J. Hazard. Mater. 2013, 250–251, 106–114. 10.1016/j.jhazmat.2013.01.051. [DOI] [PubMed] [Google Scholar]
- Adhikari S.; Sarkar D. High efficient electrochromic WO3 nanofibers. Electrochim. Acta 2014, 138, 115–123. 10.1016/j.electacta.2014.06.062. [DOI] [Google Scholar]
- Zhang L. J.; Li S.; Liu B. K.; Wang D. J.; Xie T. F. Highly efficient CdS/WO3 photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic H2 evolution under visible light. ACS Catal. 2014, 4, 3724–3729. 10.1021/cs500794j. [DOI] [Google Scholar]
- Luo J.; Zhou X.; Ma L.; Xu X. Enhanced visible-light-driven photocatalytic activity of WO3/BiOI heterojunction photocatalysts. J. Mol. Catal. A: Chem. 2015, 410, 168–176. 10.1016/j.molcata.2015.09.019. [DOI] [Google Scholar]
- Cui L.; Ding X.; Wang Y.; Shi H.; Huang L.; Zuo Y.; Kang S. Facile preparation of Z-scheme WO3/gC3 N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. 10.1016/j.apsusc.2016.07.055. [DOI] [Google Scholar]
- Li F.; Zhang L.; Chen X.; Liu Y. L.; Xu S. G.; Cao S. K. Synergistically enhanced photocatalytic reduction of CO2 on N–Fe codoped BiVO4 under visible light irradiation. Phys. Chem. Chem. Phys. 2017, 19, 21862–21868. 10.1039/c7cp03995f. [DOI] [PubMed] [Google Scholar]
- Chen L.; Meng D.; Wu X.; Wang A.; Wang J.; Wang Y.; Yu M. In situ synthesis of V4+ and Ce3+ self-doped BiVO4/CeO2 heterostructured nanocomposites with high surface areas and enhanced visible-light photocatalytic activity. J. Phys. Chem. C 2016, 120, 18548–18559. 10.1021/acs.jpcc.6b04131. [DOI] [Google Scholar]
- Zheng J. Y.; Song G.; Hong J.; Van T. K.; Pawar A. U.; Kim D. Y.; Kim C. W.; Haider Z.; Kang Y. S. Facile fabrication of WO3 nanoplates thin films with dominant crystal facet of (002) for water splitting. Cryst. Growth Des. 2014, 14, 6057–6066. 10.1021/cg5012154. [DOI] [Google Scholar]
- Hong S. J.; Lee S.; Jang J. S.; Lee J. S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. 10.1039/c0ee00743a. [DOI] [Google Scholar]
- Lee M. G.; Kim D. H.; Sohn W.; Moon C. W.; Park H.; Lee S.; Jang H. W. Conformally coated BiVO4 nanodots on porosity-controlled WO3 nanorods as highly efficient type II heterojunction photoanodes for water oxidation. Nano Energy 2016, 28, 250–260. 10.1016/j.nanoen.2016.08.046. [DOI] [Google Scholar]
- Wang M.; Che Y.; Niu C.; Dang M.; Dong D. Effective visible light-active boron and europium co-doped BiVO4 synthesized by sol–gel method for photodegradion of methyl orange. J. Hazard. Mater. 2013, 262, 447–455. 10.1016/j.jhazmat.2013.08.063. [DOI] [PubMed] [Google Scholar]