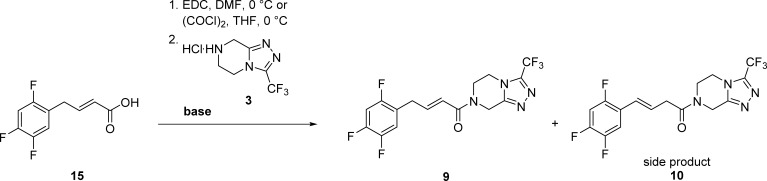
Table 2. Base Screening for Amidation to Alkene Impurity I (9).
| entry | basea | activating reagent | time(h) | temperature | overall yielda,b9 + 10 (%) | selectivity ratio 9:10c |
|---|---|---|---|---|---|---|
| 1 | NMM | EDC | 1 | 0 °C | 53 | 31.9 |
| 2 | NMM | EDC | 2 | r.t. | 77 | 30.4 |
| 3 | Et3N | EDC | 1 | 0 °C | 67 | 17.5 |
| 4 | Et3N | EDC | 2 | r.t. | 61 | 15.5 |
| 5 | Py | EDC | 2 | r.t. | 43 | 26.2 |
| 6 | DMAP | EDC | 2 | r.t. | 39 | 11.1 |
| 7 | NMM/DMAP = 9/1 | EDC | 1 | 0 °C | 85 | 20.5 |
| 8 | NMM/DMAP = 9/1 | EDC | 2 | r.t. | 75 | 17.0 |
| 9 | NMM/DMAP = 1/1 | EDC | 1 | 0 °C | 57 | 15.8 |
| 10 | NMM/DMAP = 1/1 | EDC | 2 | r.t. | 54 | 14.8 |
| 11 | NMM/Py = 1/1 | EDC | 1 | 0 °C | 44 | 18.9 |
| 12 | NMM/Py = 1/1 | EDC | 2 | r.t. | 42 | 18.7 |
| 13 | NMMb | (COCl)2 | 1 | 0 °C | 84 | 30.8 |
| 14 | Et3Nb | (COCl)2 | 1 | 0 °C | 91 | 23.6 |
| 15 | Pyb | (COCl)2 | 2 | 0 °C | 70 | 13.4 |
Reaction conditions by coupling reagent method: (i) 15 (0.50 mmol, 0.108 g), DMF (5 mL), 0 °C, EDC × HCl (0.50 mmol, 0.096 g), 0.5 h; (ii) 3 × HCl (0.5 mmol, 0.114 g), base (0.5 mmol), 0 °C for 1 h, then r.t. for 1 h. By acid chloride method: (i) 15 (0.46 mmol, 0.10 g), CH2Cl2, oxalyl chloride (0.92 mmol, 0.079 mL), DMF (cat.), 0 °C, 1.5 h; (ii) 3 × HCl (0.46 mmol, 0.105 g), base (0.92 mmol), THF (5 mL), 0 °C, 1 h.
Determined by HPLC.