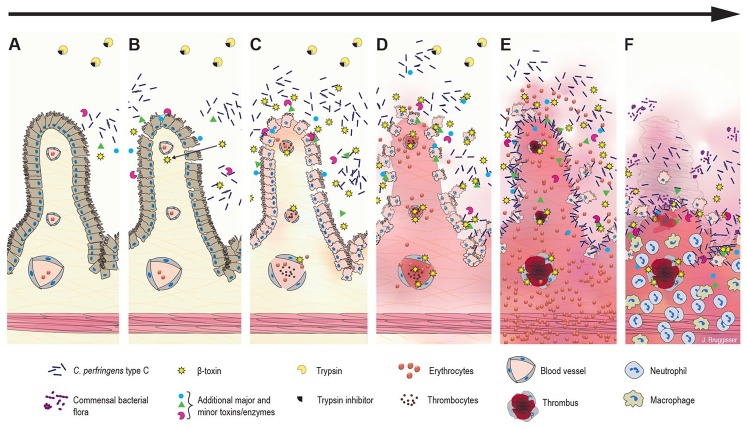
Figure 4.
Hypothesized pathogenesis of Clostridium perfringens type C enteritis in pigs. A. The disease starts with colonization, rapid proliferation of C. perfringens type C, and toxin secretion in the jejunal lumen. B. Initial epithelial damage or irritation could be caused by secreted toxins and/or the altered luminal environment. Trypsin inhibitors in the colostrum prevent degradation of C. perfringens beta-toxin (CPB), which can pass the epithelial barrier and diffuse into the lamina propria. C. CPB-induced damage in endothelial cells rapidly leads to increased permeability of vessels in the lamina propria with extravasation of fluid, plasma proteins, and erythrocytes. CPB additionally could affect platelet function and interfere with primary hemostasis promoting vascular leakage. The epithelial barrier is further disrupted by necrosis of epithelial cells. Blood components and necrotic cells accumulate in the intestinal lumen. D. Clostridial growth and toxin production accelerate. A vicious cycle of CPB-induced hemorrhage, tissue necrosis, accelerated clostridial growth, and toxin secretion develops. E. The peracute-to-acute stage of the lesion can develop within hours after initial effects: hemorrhage and necrosis rapidly progress from the initial site of damage in the jejunum. Next, there is widespread vascular thrombosis in the lamina propria and submucosa. In addition, toxins from the intestinal lumen can be absorbed into the systemic circulation and cause enterotoxemia. F. The subacute stage of the disease develops in cases in which the initial acute damage was less severe and did not cause death. Neutrophils infiltrate the zone under the necrotic mucosa. Additional bacteria present in the intestinal tract can proliferate, leading to mixed bacterial cultures.