Abstract

Carbon quantum dots (CQDs) were prepared by a chemical oxidation method using luffa sponge based activated carbon fiber as the raw material. The obtained CQDs were well characterized. The fluorescence quenching effect of Cr(VI) ion on CQDs was investigated. The results show that the addition of Cr(VI) changes the intensity of the ultraviolet characteristic absorption peak of CQDs, and causes static quenching of the fluorescence of CQDs. With the increase in the Cr(VI) concentration, the fluorescence of CQDs was gradually extinguished linearly.
Introduction
Cr is one of the main heavy metal elements that pollute the environment and affects human health.1 Usually, the toxicity of Cr is related to its valence state; Cr(VI) is 100 times more toxic than Cr(III).2 Cr(VI) is widely found in common industrial wastewaters because of its use in the electroplating, metallurgy, printing, and dyeing and chemical industries, especially in various solutions from the vanadium hydrometallurgical industry.3 Additionally, Cr(VI) discharged into environmental water bodies directly cannot be biodigested without the standard treatment. Under biomagnification, Cr(VI) is enriched into the human body through the atmosphere, water body, and food chain, which can lead to the occurrence of cancer lesions.4 Therefore, Cr(VI) has become a key monitoring target in environmental monitoring in various countries, and the measurement method for Cr(VI) has received much attention from environmental monitors.5 The rapid and sensitive identification and measurement of Cr(VI) are very important for environmental protection and human health.6
At present, the determination methods of Cr(VI) concentration mainly include complex metric titration, electrochemical analysis, high-performance liquid chromatography, ion chromatography, atomic absorption spectrometry, and mass spectrometry.7,8 Although these methods are sensitive, they have disadvantages such as a cumbersome pretreatment process for the sample to be tested, the inability of miniaturizing the detection instrument, and inability to perform time-resolved measurements.9 Compared with this method, the fluorescent probe method has the advantages of simple sample pretreatment, fast response, wide linear dynamic range, less spectral interference, and high sensitivity and has become a common analytical method for the rapid detection of metal ions.10−14 Among the fluorescent probes, carbon quantum dot fluorescent probes are easy to synthesize and cost less and their fluorescence can be adjusted. The application of carbon quantum dots (referred to as CQDs) in the field of Cr(VI) detection has become a research hotspot at home and abroad in recent years.15−20 At present, various precursors have been employed to prepare CQD, including carbon-based materials, synthetic chemicals, plant extracts, and animal byproducts.21−24
Usually, oxidant or expensive equipment is needed to prepare CQDs when carbon-based materials were used as the carbon source. The common oxidant is nitric acid and sulfuric acid, which could cause great harm to the environment.25−27 Therefore, the green preparation method of CQDs, especially, environmental-friendly oxidant and simple cut method using carbon-based materials should be developed.
In this study, CQDs were prepared using luffa sponge based activated carbon fiber (referred to as LSACF) as raw materials. To the best of our knowledge, this is the first report using ACF as the precursor. In addition, CQDs were synthesized by chemical oxidation using H2O2 as the environmental oxidant. The obtained CQDs were characterized by transmission electron microscopy (TEM), X-ray diffraction (XRD), Raman spectroscopy, and Fourier transform infrared (FTIR) spectroscopy. The morphology, chemical composition, functional groups, and fluorescence properties of the quantum dots, combined with the UV–vis absorption and fluorescence emission spectra of Cr(VI) and CQDs mixtures, were investigated to explore the effect of Cr(VI) on the fluorescence properties of CQDs. On this basis, a method of identifying and quantifying Cr(VI) using CQDs as fluorescent probes was established, which lays the foundation for rapid and highly sensitive detection of Cr(VI).
Results and Discussion
Morphological Characterization
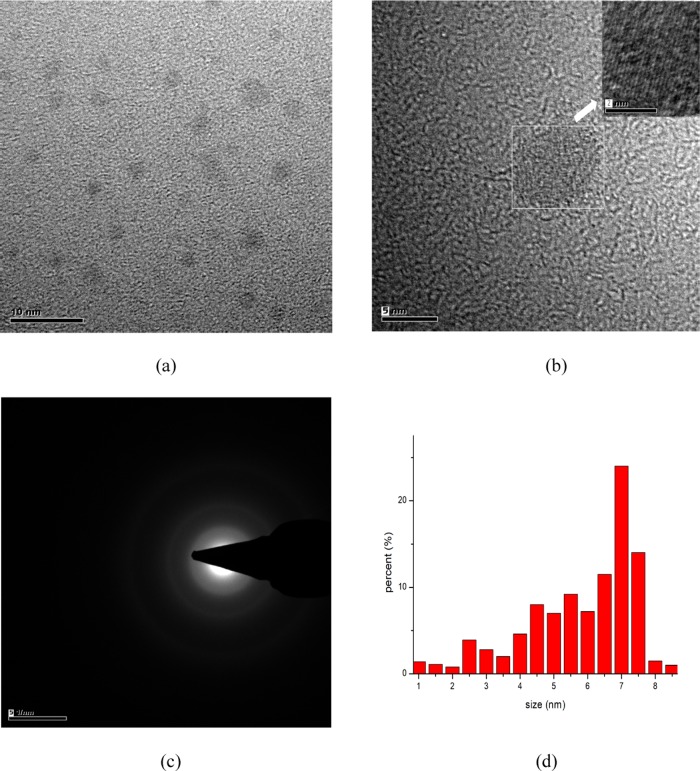
Figure 1 a shows the transmission electron micrograph of the CQDs. The CQDs were nearly spherical with good dispersion. Figure 1b shows the crystal lattice of CQDs with a lattice spacing of 0.27 nm, which is consistent with previous studies.28,29Figure 1c shows the diffused ring pattern of the CQDs, which identifies the amorphous phase of carbon of the CQDs.30 The particle sizes of the CQDs were measured using dynamic light scattering (DLS) with the dominant size from 4 to 8 nm, as shown in Figure 1d.
Figure 1.
(a) TEM image, (b) HRTEM image (inset: lattice spacing), (c) SAED patterns, and (d) particle size distribution (DLS) of CQDs.
Photoluminescence and Spectroscopic Characterization
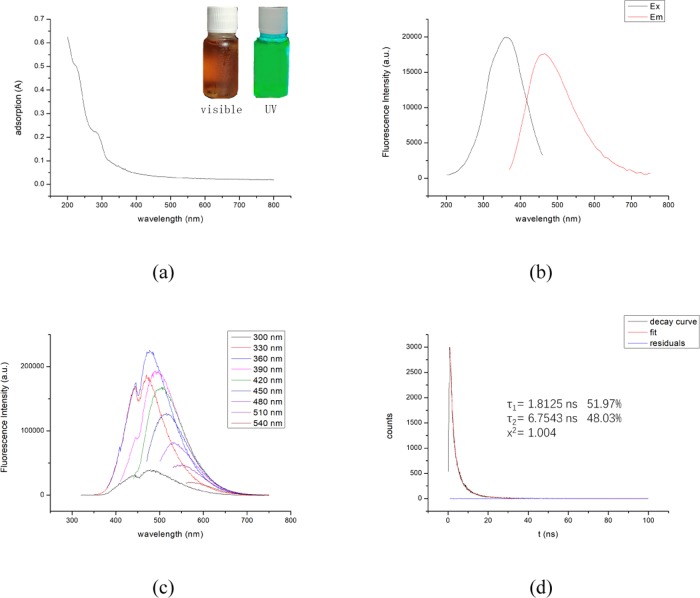
Figure 2a shows the UV–vis spectrum of the CQDs. Two shoulder absorption peaks can be observed at 238 nm, corresponding to an aromatic C=C bond and the associated π–π* transition and an approximately 290 nm due to the n−π* transition of the aromatic sp2 (C=C) domains,31 which indicates the formation of CQDs. In the inset, the CQDs became brown under the excitation of light while emitting bright green light under the excitation of ultraviolet light. The result is different from some previous studies. Mewada et al. prepared CQDs with an average size of 7.5 nm using an aqueous extract of Trapa bispinosa peel as a carbon source that emits blue light under ultraviolet light excitation.32 Arul et al. prepared CQDs with an average size of 2.5 nm using Hylocereus undatus as a carbon source with sizes ranging from 5 to 10 nm, and the same character was obtained.33 Currently, the relatively reliable theories for this result are quantum confinement theory and defective field emission.34,35
Figure 2.
(a) UV–vis spectrum (inset: picture of carbon quantum dot solution under visible light (left) and UV light (right)), (b) full spectra of excitation and emission, (c) emission spectra obtained at different excitation wavelengths, and (d) fluorescence decay lifetime of CQDs under 360 nm excitation wavelengths.
Figure 2b shows the full spectra of excitation and emission of CQDs. The fluorescence intensity maximum was obtained with excitation at 360 nm. Figure 2c shows the emission spectra obtained at different excitation wavelengths for the CQDs. It is shown that a red shift occurs as the excitation wavelength is increased and the maximum fluorescence intensity is reached with excitation at 360 nm. The luminescence mechanism of the excitation wavelength dependence is still unclear. It is generally believed that the luminescence is derived from a defect existing on the surface of the CQDs, which acts as an excitation energy trap and emits light under the excitation of a certain wavelength of light.
Figure 2d shows the fluorescence decay lifetime of the CQDs under 360 nm excitation. The CQDs have two fluorescence decay lifetimes of 1.8 and 6.7 ns, and further calculation shows 4.1 ns is the average fluorescence decay lifetime. Using quinine sulfate (54%, 0.1 mol/L H2SO4) as reference material, the fluorescence quantum yield of the CQDs was determined to be 2.1%, which is lower than that of CQDs prepared by others.36,37 The possible reason is related to the lack of incorporating a passivating agent and heteroatoms in the preparation process.
Structure Characterization
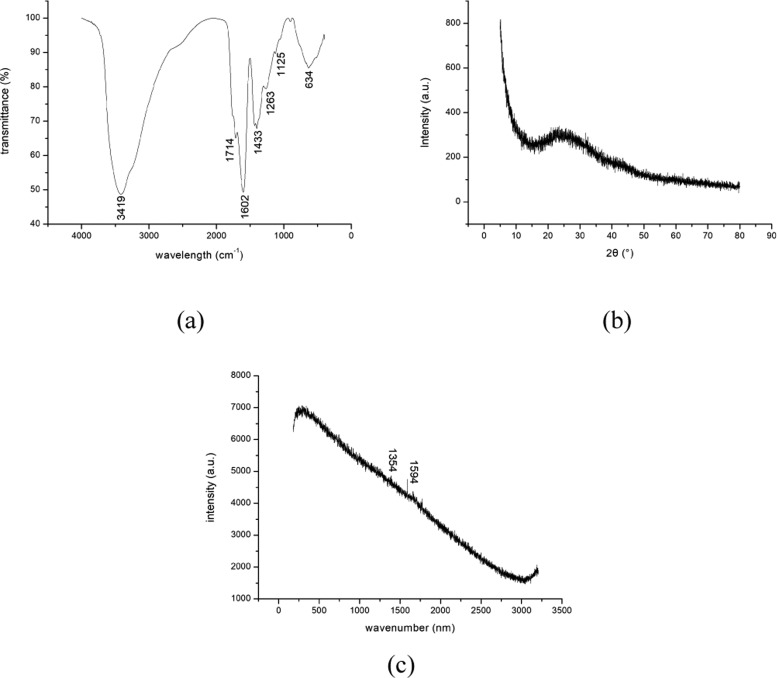
The functional groups on CQDs surface were analyzed using the FTIR analysis. Figure 3a shows the FTIR spectrum of the CQDs. A broad band at 3419 cm–1 corresponds to the −OH stretching vibration peak.38 The peak at 1715 is ascribed to the C=O group. The peaks at 1602 and 1433 cm–1 are assigned to the −COO group. The appearance of peaks at 1263 and 1165 cm–1 is due to C–O–C stretching vibration group. A C–C stretching vibration peak appears at 634 cm–1. The presences of −COOH and −OH groups provides the water solubility of the CQDs.
Figure 3.
(a) FTIR, (b) XRD, and (c) Raman spectra of CQDs.
Figure 3b shows the XRD pattern of the CQDs. A distinct broad absorption peak appeared at approximately 27.6°, indicating the amorphous carbon structure of the CQDs. In addition, there is also a very small diffraction peak at 44°, corresponding to the (100) crystal plane of graphite, which means that the carbon fiber has a certain degree of graphitization.
Figure 3c shows the Raman spectrum of the CQDs. Generally, the intensities of the D peak (at approximately 1354 cm–1) and G peak (at approximately 1594 cm–1) are employed to determine the degree of graphitization of a sample. However, the Raman spectrum of the CQDs shows that G peak (indicating crystallinity) and D peak (indicating the degree of disorder) are not significant, which is caused by the interference of the intense fluorescence from the carbon quantum dots.39,40 Therefore, no certain result was obtained from the Raman spectra of CQDs.
Detection of Cr(VI) Ions
To study the application of the prepared CQDs as a metal fluorescent probe for Cr(VI), Cr(VI) ions were used to test their effect on the PL value of the CQDs. Besides, other metal ions (K+, Mg2+, Zn2+, Cr3+, Na+, Al3+, and Ca2+) were selected to study their effect on the PL value of the CQDs.
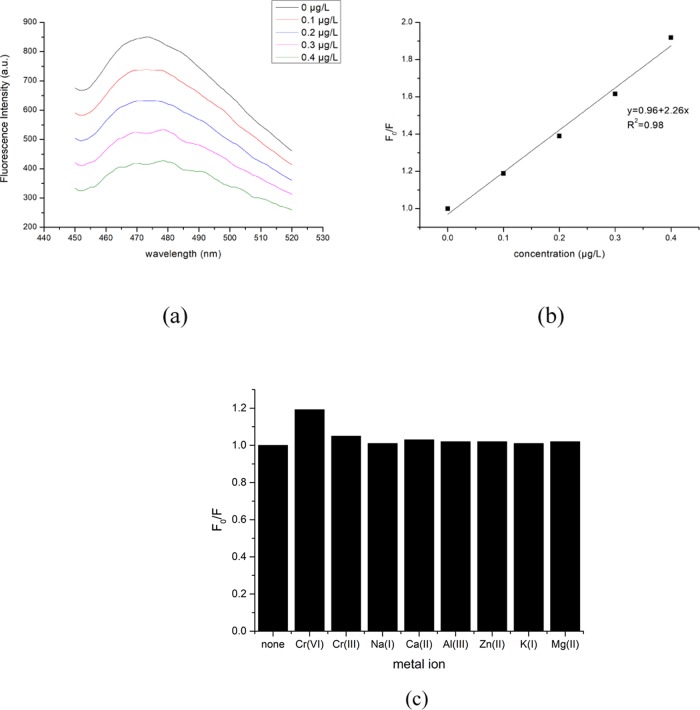
Figure 4a shows the emission spectra obtained at different Cr(VI) concentrations. As the Cr(VI) concentration increased from 0 to 0.4 μg/L, the fluorescence intensity of the CQDs decreased gradually at 473 nm emission, which indicates that Cr(VI) has the fluorescence quenching effect on the CQDs.
Figure 4.
(a) Emission spectra obtained at different Cr(VI) concentrations, (b) the normal curve of F0/F under excitation at 360 nm, and (c) the effect of other metal ions on F0/F.
Figure 4b shows the normal curve of F0/F for the CQDs. F0 is the fluorescence intensity of the CQDs at 473 nm emission with no addition of Cr(VI) and F is the fluorescence intensity of the CQDs at 473 nm emission with different concentrations of Cr(VI). The obtained linear correlation coefficient R2 is 0.989, which indicates a good linear relationship between the fluorescence intensity of the CQDs and the concentration of Cr(VI). The results show the promising potential of the CQDs for a fast and easy determination of Cr(VI). Balavigneswaran et al used self-made silver nanoparticles (AgNPs) as the probe to quickly detect the concentration of Cr(VI), and the obtained results were good. However, AgNPs are a kind of metal material and have a relatively high cost so that extensive use of this material is difficult.41 Zheng et al. developed salicylaldehyde rhodamine B hydrazone (SRBH) as a new spectrofluorometric probe to detect the concentration of Cr(VI), and a good linear relationship between the concentrations of (SRBH) and Cr(VI) was achieved.42 However, the additional H2SO4 was significantly necessary to enhance the blank fluorescence of probe. Therefore, the CQDs used in this study show the advantages of simple operation and environmental-friendly material.
Figure 4c shows the effect of other metal ions (K+, Mg2+, Zn2+, Cr3+, Na+, Al3+, and Ca2+) on the PL value of the CQDs. The results demonstrate the negligible effect of these metal ions on the PL value of the CQDs, which confirms the availability of CQDs to determine the Cr(VI) concentration.
The fluorescence quenching mechanism of Cr(VI) on CQDs has been investigated. The inner filter effect (IFE) is employed to explain the fluorescence quenching mechanisms of CQDs. As reported in previous literature, Cr(VI) exhibits broad absorption at 260, 360, and 440 nm, respectively.43 The excitation spectrum of LSACF CQDs has the obvious excitation band at 360 nm and the emission band at 473 nm under the excitation of 360 nm. There is an obvious overlapping in the excitation spectrum, indicating that Cr(VI) can shield the excitation light for CQDs. Therefore, the increasing Cr(VI) concentration could lead to the more strong fluorescence quenching of CQDs, which ensures that the IFE occurs in a highly efficient way.43
In addition, the quenching effect kinetic mechanism was been investigated. Generally, fluorescence quenching usually is caused by the static quenching effect (SQE), or the dynamic quenching effect (DQE), or both.44 The ground-state complex formation model of DQE and SQE can be described by the Stern–Volmer equation (eq 1)
| 1 |
where KSV is the quenching constant, τ0 is the average lifetime of CQDs (4.1 ns), and Kq is the quenching rate constant. For Cr(VI) sensing, Kq was calculated to be 6.86 × 1012 M–1 s–1. The obtained Kq is much higher than the maximum scatter collision quenching constant (1.0 × 1010 M–1 s–1), which indicates a static quenching.
Conclusions
In this study, CQDs were prepared by chemical oxidation using LSACF. The obtained CQDs have a uniform particle size with an average particle size of 6.8 nm and good solubility. The PL intensity of the CQDs depends on excitation wavelength and the CQDs emit green light under 365 nm UV light. In addition, the application of CQDs as a metal probe was tested using Cr(VI) as the target metal. As the Cr(VI) concentration increased, the fluorescence quenching rate of the CQDs increased. At present, the CQDs prepared using LSACF still have some shortcomings, such as obvious aggregation and low quantum yield, which still need to be improved in the future.
Experimental Section
Materials and Instruments
Luffa sponge was purchased from Luohe Huahui Daily Necessities Co., Ltd. All of the reagents employed, including hydrogen peroxide, potassium dichromate, calcium chloride, chromium trichloride, zinc chloride, magnesium chloride, potassium chloride, aluminum chloride, and sodium chloride, were analytical grade and applied without any further purification. An aqueous Cr(VI) solution was prepared using distilled water.
The TEM measurements were performed using a high-resolution transmission electron microscope (JEM-2100F). The RS measurements were performed using a confocal microscopic Raman spectrometer (InVia). The PL measurements were performed using a spectrofluorometer (Sigma). The spectrophotometric measurements were measured using a UV–vis spectrometer (UNICOWFUV-2). The XRD analysis was performed using an X-ray diffractometer (D8 Advance). FTIR analysis was performed using a Fourier transform infrared spectrometer (Nexus). The dynamic light scattering (DSL) test was performed using a dynamic light scattering particle size analyzers (NANOZS). Other general instruments included an electronic balance (BS223S), a tubular resistance furnace (SK2-4-12A), a centrifuge (TG16-II), a drying box (DGG-9123A), a magnetic stirrer (TWCL-D), a lyophilizer (JL-D10N-50C), and an oil bath (HH-1S).
Preparation of LSACF
The luffa sponge was oxidized, carbonized, and activated by a high-temperature tubular resistance furnace. The heating process of the resistance furnace is as follows: heating at 8 °C/min to 200 °C for 120 min, then heating to 750 °C for 70 min, and then cooling naturally; the resistance furnace was fed with N2 (flow rate is 0.7 L/min) as a protective gas; the obtained product was luffa sponge activated carbon fiber (LSACF).
Preparation of LSACF-Based CQD
Two grams of LSACF powder was added to a round-bottom flask followed by 100 mL of hydrogen peroxide (15%) and sonication for 30 min before heating in an oil bath for 24 h at 100 °C. The round-bottom flask was removed after the reaction, naturally cooled, and filtered using a 0.45 μm polyethersulfone membrane. The filtrate was centrifuged in a centrifuge for 15 min at 10 000 rpm, and then the supernatant was again filtered through a 0.22 μm needle-type syringe filter. The obtained filtrate was dialyzed into a dialysis bag with a molecular weight of 2000 d. The dialysis time was 2 days, with changing water intervals of 24 h. After the completion of the dialysis, the freeze-dried solid powder is the desired CQDs.
Calculation of Quantum Yield (QY) and Fluorescence Decay Lifetime
Determination of fluorescence quantum yield: quantum yield was determined by the reference method. Quinoline sulfate (referred to as QS) with 55% QY was used as a reference standard. The fluorescence integral area and absorbance value (A) of carbon quantum dots (CQD) and quinoline sulfate solutions were measured at the same excitation wavelength. The fluorescence quantum yield of the CQDs was obtained according to the following eq 2
| 2 |
where QY is the fluorescence quantum yield in %, I is the fluorescence integral area, A is the absorbance of the incident light, and n is the refractive index of the solvent. The fluorescence quantum yield of the CQDs was determined to be 2.1%.
The exponential decay curve was fitted to calculate the fluorescence decay lifetime. The following eq 3 was employed in the fitting45
| 3 |
where A is the weight coefficient, τ is the fluorescence decay lifetime, and I is the fluorescence intensity. The CQDs have two fluorescence decay lifetimes of 1.8 and 6.7 ns.
Determination of Cr(VI) Using CQDs
The solid CQDs sample was diluted to the appropriate 1 mg/L using pure water, and then 2 mL of the solution including Cr(VI) ion was added to the CQDs solution in a ratio of 1:1. After mixing, the PL value of the CQDs was measured.
Acknowledgments
The authors sincerely thank the characterization from the Wuhan University of Technology Materials Research and Testing Center.
Study on Comprehensive Control of Rocky Desertification and Ecological Service Function Improvement in Karst Peaks (No. 2016YFC0502402).
The authors declare no competing financial interest.
References
- Li Y.; Zhaohui J.; Tielong L.; Shujing L. Removal of hexavalent chromium in soil and groundwater by supported nano zero-valent iron on silica fume. Water Sci. Technol. 2011, 63, 2781–2787. 10.2166/wst.2011.454. [DOI] [PubMed] [Google Scholar]
- Fu Z.; Chen W.; Jiang Z.; Macdonald B. E.; Lin Y.; Fei C.; Zhang L.; Lavernia E. J. Influence of Cr removal on the microstructure and mechanical behaviour of a high-entropy Al0.8Ti0.2CoNiFeCr alloy fabricated by powder metallurgy. Powder Metall. 2018, 106–114. 10.1080/00325899.2018.1427662. [DOI] [Google Scholar]
- Coşkun R.; Birgül H.; Delibaş A. Synthesis of functionalized PET fibers by grafting and modification and their application for Cr(VI) ion removal. J. Polym. Res. 2018, 25, 29. 10.1007/s10965-017-1429-7. [DOI] [Google Scholar]
- Feng X.; Liang C.; Yu J.; Jiang X. Facile fabrication of graphene oxide-polyethylenimine composite and its application for the Cr(VI) removal. Sep. Sci. Technol. 2018, 53, 2376–2387. 10.1080/01496395.2018.1458880. [DOI] [Google Scholar]
- Li H.; Gao P.; Cui J.; Zhang F.; Wang F.; Cheng J. Preparation and Cr(VI) removal performance of corncob activated carbon. Environ. Sci. Pollut. Res. 2018, 25, 20743–20755. 10.1007/s11356-018-2026-y. [DOI] [PubMed] [Google Scholar]
- Gong K.; Qian H.; Lu Y.; Min L.; Sun D.; Qian S.; Qiu B.; Guo Z. Ultrasonic Pretreated Sludge Derived Stable Magnetic Active Carbon for Cr(VI) Removal from Wastewater. ACS Sustainable Chem. Eng. 2018, 6, 7283–7291. 10.1021/acssuschemeng.7b04421. [DOI] [Google Scholar]
- Drinčić A.; Zuliani T.; Ščančar J.; Milačič R. Determination of hexavalent Cr in river sediments by speciated isotope dilution inductively coupled plasma mass spectrometry. Sci. Total Environ. 2018, 637–638, 1286–1294. 10.1016/j.scitotenv.2018.05.112. [DOI] [PubMed] [Google Scholar]
- Shahrivari S.; Faridbod F.; Ganjali M. R. Highly selective and sensitive colorimetric determination of Cr3+ ion by 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol functionalized Au nanoparticles. Spectrochim. Acta, Part A 2018, 191, 189. 10.1016/j.saa.2017.09.064. [DOI] [PubMed] [Google Scholar]
- Mokoena D. P.; Mngadi S. V.; Sihlahla M.; Dimpe M. K.; Nomngongo P. N. Development of a Rapid and Simple Digestion Method of Freshwater Sediments for As, Cd, Cr, Cu, Pb, Fe, and Zn Determination by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES): An Evaluation of Dilute Nitric Acid. Soil Sediment Contam. 2019, 28, 323–333. 10.1080/15320383.2019.1575334. [DOI] [Google Scholar]
- Zhang J.; Fu Y.; Mei Y.; Jiang F.; Lakowicz J. R. Fluorescent Metal Nanoshell – Probe to Detect Single miRNA in Lung Cancer Cell. Anal. Chem. 2010, 82, 4464–4471. 10.1021/ac100241f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu L.; Peng J.; Peng H.; Liu S.; Xiao H.; Dan L.; Pan Z.; Yu C.; Fang C.; Yan H. Fluorescent carbon dots for sensitive detection of Cr(VI) in aqueous media and its application in test papers. RSC Adv. 2016, 6, 95469. 10.1039/C6RA19977A. [DOI] [Google Scholar]
- Tan H.-c.; Zhao W.-h.; Qiu Q.; Zhang R.; Zuo Y.-y.; Yang L.-j. Green synthesis of nitrogen-doped fluorescent carbon quantum dots for selective detection of iron. Fullerenes, Nanotubes, Carbon Nanostruct. 2017, 25, 417–422. 10.1080/1536383X.2017.1326102. [DOI] [Google Scholar]
- Thambiraj S.; Dhesingh R. S. Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl. Surf. Sci. 2016, 390, 435–443. 10.1016/j.apsusc.2016.08.106. [DOI] [Google Scholar]
- Tyagi A.; Tripathi K.; Singh N.; Choudhary S.; Gupta R. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423. 10.1039/C6RA10488F. [DOI] [Google Scholar]
- Wang B.; Lin Y.; Tan H.; Luo M.; Dai S.; Lu H.; Huang Z. One-pot synthesis of N-doped carbon dots by pyrolyzing the gel composed of ethanolamine and 1-carboxyethyl-3-methylimidazolium chloride and their selective fluorescence sensing for Cr(VI) ions. Analyst 2018, 143, 1906–1915. 10.1039/C8AN00077H. [DOI] [PubMed] [Google Scholar]
- Gong X.; Liu Y.; Yang Z.; Shuang S.; Zhang Z.; Dong C. An “on-off-on” fluorescent nanoprobe for recognition of chromium(VI) and ascorbic acid based on phosphorus/nitrogen dual-doped carbon quantum dot. Anal. Chim. Acta 2017, 968, 85–96. 10.1016/j.aca.2017.02.038. [DOI] [PubMed] [Google Scholar]
- Vaz R.; Bettini J.; Júnior J. G. e. F.; Lima E. D. S.; Botero W. G.; Santos J. C. C.; Schiavon M. A. n. High luminescent carbon dots as an eco-friendly fluorescence sensor for Cr(VI) determination in water and soil samples. J. Photochem. Photobiol., A 2017, 346, 502–511. 10.1016/j.jphotochem.2017.06.047. [DOI] [Google Scholar]
- Carrasco P. M.; García I.; Yate L.; Zaera R. T.; Ruiz V.; et al. Graphene quantum dot membranes as fluorescent sensing platforms for Cr (VI) detection. Carbon 2016, 109, 658–665. 10.1016/j.carbon.2016.08.038. [DOI] [Google Scholar]
- Pacquiao M. R.; de Luna M. D. G.; et al. Highly fluorescent carbon dots from enokitake mushroom as multi-faceted optical nanomaterials for Cr6+ and VOC detection and imaging applications. Appl. Surf. Sci. 2018, 453, 192–203. 10.1016/j.apsusc.2018.04.199. [DOI] [Google Scholar]
- Singh V. K.; Singh V.; Kumar Y. P.; Subhash C.; Daraksha B.; Vijay K.; Biplob K.; Mahe T.; Hadi H. S. Bright-blue-emission nitrogen and phosphorus doped carbon quantum dots as a promising nanoprobe for detection of Cr (VI) and ascorbic acid in pure aqueous solution and in living cell. New J. Chem. 2018, 42, 12990–12997. 10.1039/C8NJ02126K. [DOI] [Google Scholar]
- Yang S. T.; Wang X.; Wang H.; Lu F.; Luo P. G.; Cao L.; Meziani M. J.; Liu J. H.; Liu Y.; Chen M.; et al. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 2009, 113, 18110. 10.1021/jp9085969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Zheng H.; Long Y.; Zhang L.; Gao M.; Bai W. Microwave–hydrothermal synthesis of fluorescent carbon dots from graphite oxide. Carbon 2011, 49, 3134–3140. 10.1016/j.carbon.2011.03.041. [DOI] [Google Scholar]
- Yadav P. K.; Singh V. K.; Chandra S.; Bano D.; Kumar V.; Talat M.; Hasan S. H. Green Synthesis of Fluorescent Carbon Quantum Dots from Azadirachta indica Leaves and Their Peroxidase-Mimetic Activity for the Detection of H2O2 and Ascorbic Acid in Common Fresh Fruits. ACS Biomater. Sci. Eng. 2019, 5, 623–632. 10.1021/acsbiomaterials.8b01528. [DOI] [PubMed] [Google Scholar]
- Yadav P. K.; Singh V. K.; Kumar C.; Chandra S.; Jit S.; Singh S. K.; Talat M.; Hasan S. H. A Facile Synthesis of Green-Blue Carbon Dots from Artocarpus lakoocha Seeds and Their Application for the Detection of Iron (III) in Biological Fluids and Cellular Imaging. ChemistrySelect 2019, 4, 12252–12259. 10.1002/slct.201903220. [DOI] [Google Scholar]
- Tan M.; Zhang L.; Tang R.; Song X.; Li Y.; Wu H.; Wang Y.; Lv G.; Liu W.; Ma X. Enhanced photoluminescence and characterization of multicolor carbon dots using plant soot as a carbon source. Talanta 2013, 115, 950–956. 10.1016/j.talanta.2013.06.061. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Jian Z.; Jiang Z.; Wang W.; Liu X. High-quality carbon dots: synthesis, peroxidase-like activity and their application in the detection of H 2 O 2, Ag + and Fe 3+. RSC Adv. 2014, 4, 17387–17392. 10.1039/C3RA47593J. [DOI] [Google Scholar]
- Zhang S.; Wang Q.; Tian G.; Ge H. A fluorescent turn-off/on method for detection of Cu2+and oxalate using carbon dots as fluorescent probes in aqueous solution. Mater. Lett. 2014, 115, 233–236. 10.1016/j.matlet.2013.10.086. [DOI] [Google Scholar]
- Hoan B. T.; Van P. H.; Van H. N.; Nguyen D. H.; Tam P. D.; Nguyen K. T.; Pham V. H. Luminescence of lemon-derived carbon quantum dot and its potential application in luminescent probe for detection of Mo6+ ions. Luminescence 2018, 33, 545–551. 10.1002/bio.3444. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wenxia Z.; Qianjin W.; Jiyang F. C8-structured carbon quantum dots: Synthesis, blue and green double luminescence, and origins of surface defects. Carbon 2014, 79, 165–173. 10.1016/j.carbon.2014.07.056. [DOI] [Google Scholar]
- Ahmadian-Fard-Fini S.; Salavati-Niasari M.; Ghanbari D. Hydrothermal green synthesis of magnetic Fe3O4-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim. Acta, Part A 2018, 203, 481. 10.1016/j.saa.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Xue W.; Chen H.; JM L. Peroxynitrous-acid-induced chemiluminescence of fluorescent carbon dots for nitrite sensing. Anal. Chem. 2011, 83, 8245–51. 10.1021/ac202039h. [DOI] [PubMed] [Google Scholar]
- Mewada A.; Pandey S.; Shinde S.; Mishra N.; Oza G.; Thakur M.; Sharon M.; Sharon M. Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater. Sci. Eng., C 2013, 33, 2914–2917. 10.1016/j.msec.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Arul V.; Edison T. N.; Lee Y. R.; Sethuraman M. G. Biological and catalytic applications of green synthesized fluorescent N-doped carbon dots using Hylocereus undatus. J. Photochem. Photobiol., B 2017, 168, 142–148. 10.1016/j.jphotobiol.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Andrade G. R. S.; Costa S. S. L.; Nascimento C. C.; Gimenez I. F. Synthesis of green-emitting carbon quantum dots with excitation wavelength dependent photoluminescence obtained from aqueous beetroot extract. Mrs Adv. 2016, 1, 1371–1376. 10.1557/adv.2016.195. [DOI] [Google Scholar]
- Bharathi D.; Siddlingeshwar B.; Krishna R. H.; Singh V.; Kottam N.; Divakar D. D.; Alkheraif A. A. Green and Cost Effective Synthesis of Fluorescent Carbon Quantum Dots for Dopamine Detection. J. Fluoresc. 2018, 28, 1–7. 10.1007/s10895-018-2218-3. [DOI] [PubMed] [Google Scholar]
- Na R. K.; Nafiujjaman M.; Cherukula K.; Sang J. L.; Hong S. J.; Lim H. N.; Chan H. P.; Park I. K.; Lee Y. K.; Kwon I. K. Microwave-Assisted Synthesis of Biocompatible Silk Fibroin-Based Carbon Quantum Dots. Part. Part. Syst. Charact. 2017, 35, 1700300 10.1002/ppsc.201700300. [DOI] [Google Scholar]
- Liu Y.; Liu C.; Zhang Z. Synthesis of highly luminescent graphitized carbon dots and the application in the Hg2+ detection. Appl. Surf. Sci. 2012, 263, 481–485. 10.1016/j.apsusc.2012.09.088. [DOI] [Google Scholar]
- Bhaisare M. L.; Pandey S.; Khan M. S.; Talib A.; Wu H. F., Fluorophotometric Determination of Critical Micelle Concentration (CMC) of Ionic and Non-Ionic Surfactants with Carbon Dots Based on the Stokes Shift. Talanta 2015, 108 ( (2), ), 480a. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Hu L.; Zhu K.; Yan M.; Liu J.; Yang J.; Bi H. Contribution of oligomer/carbon dots hybrid semiconductor nanoribbon to surface-enhanced Raman scattering property. Appl. Surf. Sci. 2016, 364, 660–669. 10.1016/j.apsusc.2015.12.214. [DOI] [Google Scholar]
- Zhu S.; Meng Q.; Wang L.; Zhang J.; Song Y.; Jin H.; Zhang K.; Sun H.; Wang H.; Yang B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem., Int. Ed. 2013, 52, 3953–3957. 10.1002/anie.201300519. [DOI] [PubMed] [Google Scholar]
- Balavigneswaran C. K.; Kumar T. S. J.; Packiaraj R. M.; Prakash S. Rapid detection of Cr(VI) by AgNPs probe produced by Anacardium occidentale fresh leaf extracts. Appl. Nanosci. 2014, 4, 367–378. 10.1007/s13204-013-0203-3. [DOI] [Google Scholar]
- Zheng A. F.; Chen J. L.; Wu G.; Wu G.; Yuan G. Z.; Wei H. P. A novel fluorescent distinguished probe for Cr (VI) in aqueous solution. Spectrochim. Acta, Part A 2009, 74, 265–270. 10.1016/j.saa.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Xie Z.; Qu D.; Li D.; Du P.; Jing X.; Sun Z. On–Off–On Fluorescent Carbon Dot Nanosensor for Recognition of Chromium(VI) and Ascorbic Acid Based on the Inner Filter Effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. 10.1021/am4042355. [DOI] [PubMed] [Google Scholar]
- Tang M.; Liu X.; Zhang N.; Pang J.; Chen L.; et al. Synthesis of nitrogen and sulfur doped carbon dots and application for fluorescence detection of Cd(II) in real water samples. Anal. Methods 2019, 5214. 10.1039/C9AY01658A. [DOI] [Google Scholar]
- Magde D.; Wong R.; Seybold P. G. Fluorescence Quantum Yields and Their Relation to Lifetimes of Rhodamine 6G and Fluorescein in Nine Solvents: Improved Absolute Standards for Quantum Yields¶. J. Photochem. Photobiol. 2002, 75, 327–334. . [DOI] [PubMed] [Google Scholar]