FIG 1.
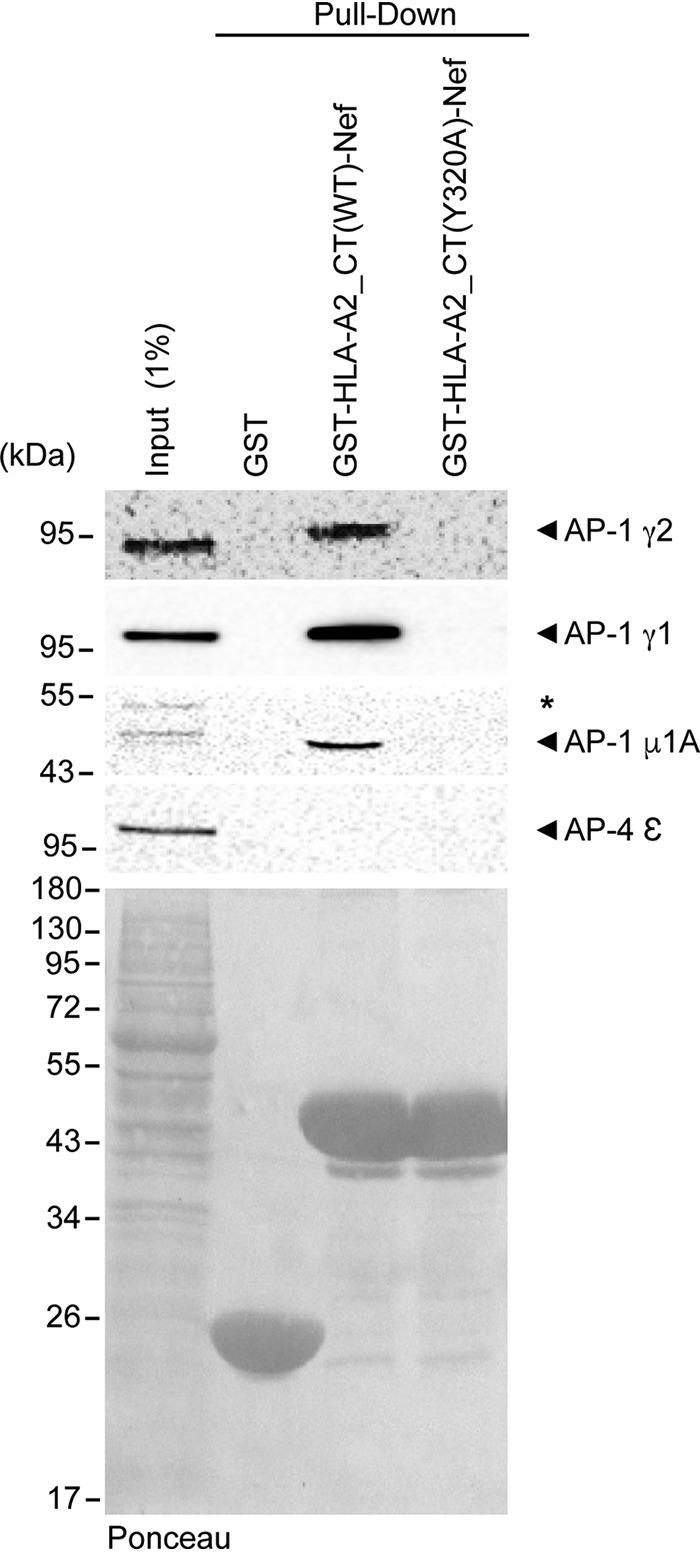
AP-1γ2 interacts with HLA-A2_CT-Nef through the noncanonical tyrosine motif of HLA-A2_CT. Immunoblots for AP-1γ2, AP-1γ1, AP-1μ1A, and AP-4ε indicate that the AP-1 subunits γ2, γ1, and μ1A interact with a chimera of Nef and the cytosolic tail of HLA [GST-HLA-A2_CT(WT)-Nef] and that this interaction is inhibited by a point mutation in the Tyr320 residue of HLA-A2 [GST-HLA-A2_CT(Y320A)-Nef]. AP-4ε was used as a negative control for the interaction. GST-HLA-A2_CT(WT)-Nef and GST-HLA-A2_CT(Y320A)-Nef were produced in E. coli, immobilized onto glutathione beads, and incubated with cleared HeLa cell lysates. One percent of the total protein lysate incubated with the beads was used as the input. As a loading control, the nitrocellulose membrane was stained with Ponceau S (bottom).
