Figure 5. Membrane-proximal Regions of δ2-Protocadherins Lack cis Interface Signatures.
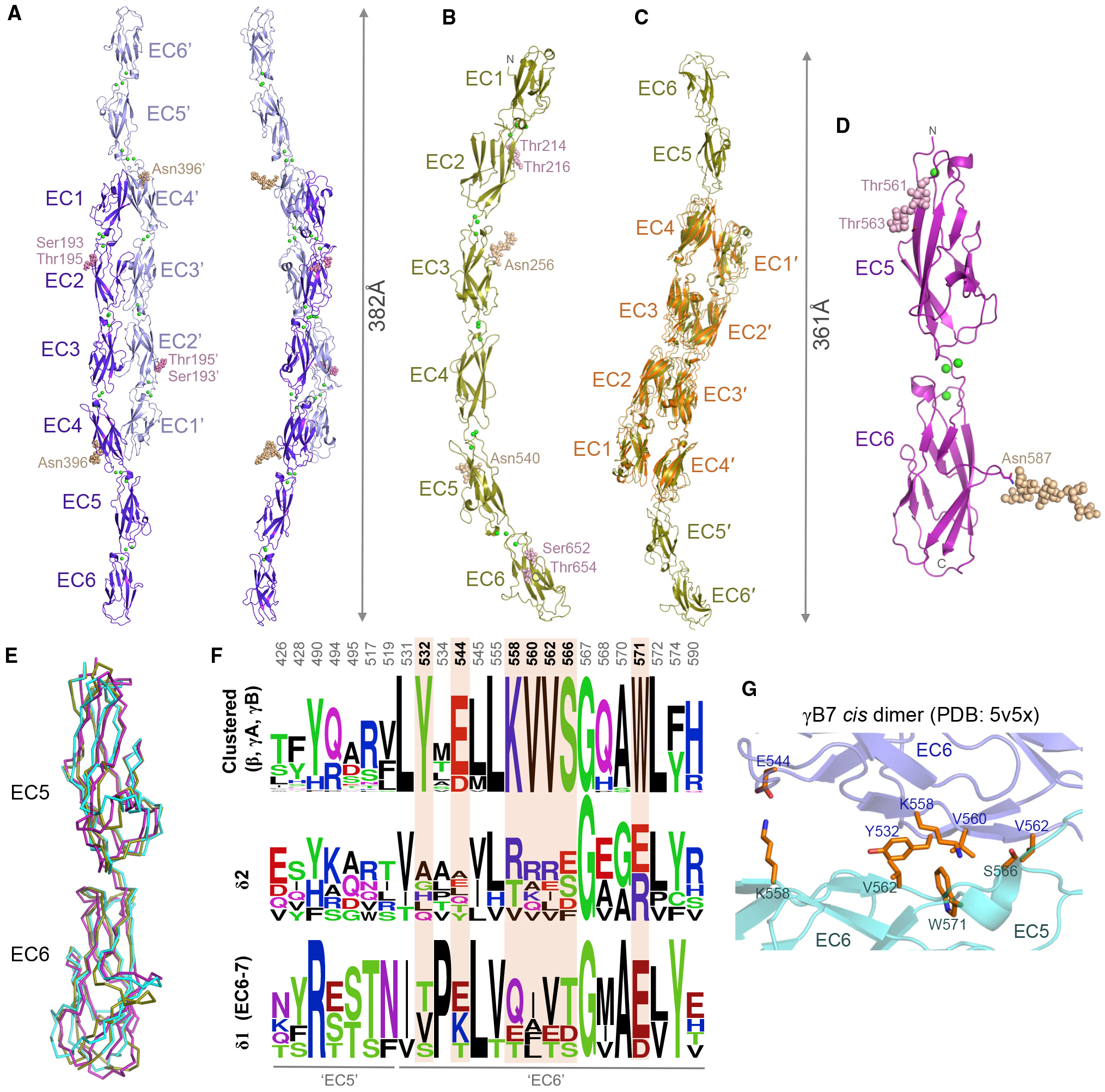
(A and B) Crystal structures of Xenopus pcdh 8.1 EC1–EC6 (A) and human pcdh10 EC1–EC6 (B), shown as ribbons. Green spheres: calcium ions; wheat and magenta spheres: N- and O-linked glycans.
(C) Superposition of two molecules of pcdh10 EC1–EC6 (gold) over the trans dimer structure of pcdh10 EC1–EC4 (orange).
(D) Crystal structure of human pcdh8 EC5–EC6 membrane-proximal fragment, shown as ribbon.
(E) Superposition of EC5–EC6 membrane-proximal regions of pcdh8 (magenta) and pcdh10 (gold) over EC5–EC6 from clustered pcdh γB7 (cyan, PDB: 5V5X; Goodman et al., 2017).
(F) Sequence logo plots of aligned mouse clustered protocadherins β, γA, and γB (top) or human δ-protocadherins (bottom). Only residue positions with side chains > 20% buried in the γB7 cis dimer (PDB: 5V5X) are shown. Positions conserved only in clustered protocadherinpcdhs are highlighted orange. Numbering refers to pcdh γB7.
(G) Close-up view of the cis interface of clustered pcdh γB7 (Goodman et al., 2017) showing differentially conserved interface residues from (E). Protomers colored slate and cyan.
