Abstract
A 65-year-old man was admitted to our hospital due to an abnormal shadow on chest radiographs. Chest computed tomography (CT) revealed a tumor (diameter: 38 mm × 27 mm) and another small nodule in the left upper lobe of the lung, which were accompanied by lymphangitis of the left upper lobe. The patient underwent a transbronchial lung biopsy, following which he was diagnosed with lung adenocarcinoma. Contrast-enhanced CT and ultrasound imaging revealed bilateral pulmonary artery thrombosis and multiple venous thromboses. He was thus diagnosed with stage IIB lung cancer complicated by Trousseau’s syndrome. Chemotherapy was initiated using platinum doublets, while infusions of unfractionated heparin and Xa inhibitor were administered for anticoagulant therapy. Following chemotherapy, the main tumor had shrunk, and his lymphangitis, pulmonary artery thrombosis, and multiple venous thromboses had resolved. We then could perform a left upper lobectomy and lymph node dissection safely.
Keywords: Salvage surgery, lung cancer, Trousseau’s syndrome, chemotherapy, adenocarcinoma, thrombosis
Introduction
Trousseau’s syndrome, first described in 1865, is a paraneoplastic syndrome in which thromboembolism is complicated by malignant tumors (1,2). Prognosis is poor among patients with lung cancer complicated by Trousseau’s syndrome (3,4). Here, we discuss a rare case of Trousseau’s syndrome in a patient with lung adenocarcinoma. As surgery was deemed unsafe of sudden death, we first administered chemotherapy and anticoagulant therapy, following which salvage surgery was successful.
Case presentation
A 65-year-old man with hypertension and a history of cerebellar infraction was admitted to our hospital due to an abnormal shadow on chest radiographs. Although he had no subjective symptoms, chest computed tomography (CT) revealed a tumor (diameter: 38 mm × 27 mm) and another small nodule in the left upper lobe, which were accompanied by lymphangitis of the left upper lobe (Figure 1). Following a transbronchial lung biopsy, the patient was diagnosed with lung adenocarcinoma. However, chest CT also revealed bilateral pulmonary artery thrombosis and thrombosis of the left femoral vein (Figure 2A,B).
Figure 1.
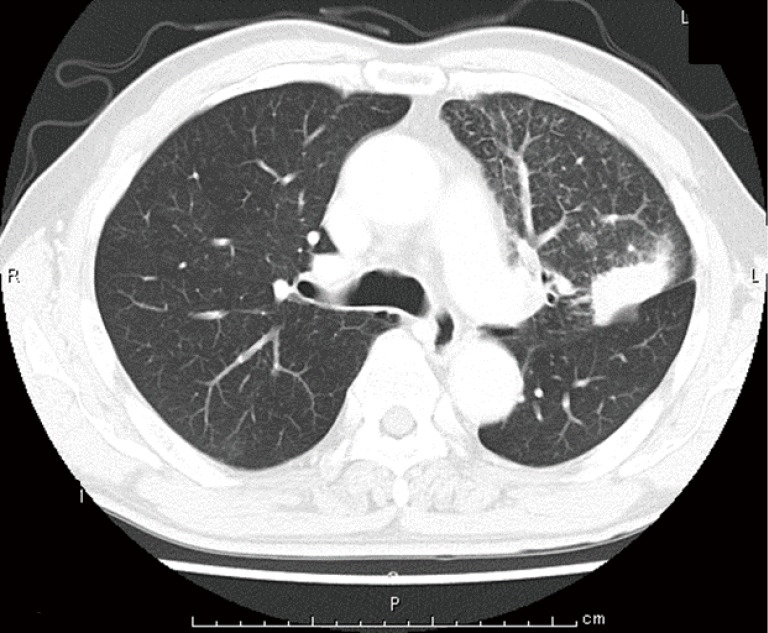
Chest computed tomography (CT) revealed a tumor (diameter: 38 mm × 27 mm) and another small nodule in the left upper lobe, which were accompanied by lymphangitis of the left upper lobe.
Figure 2.
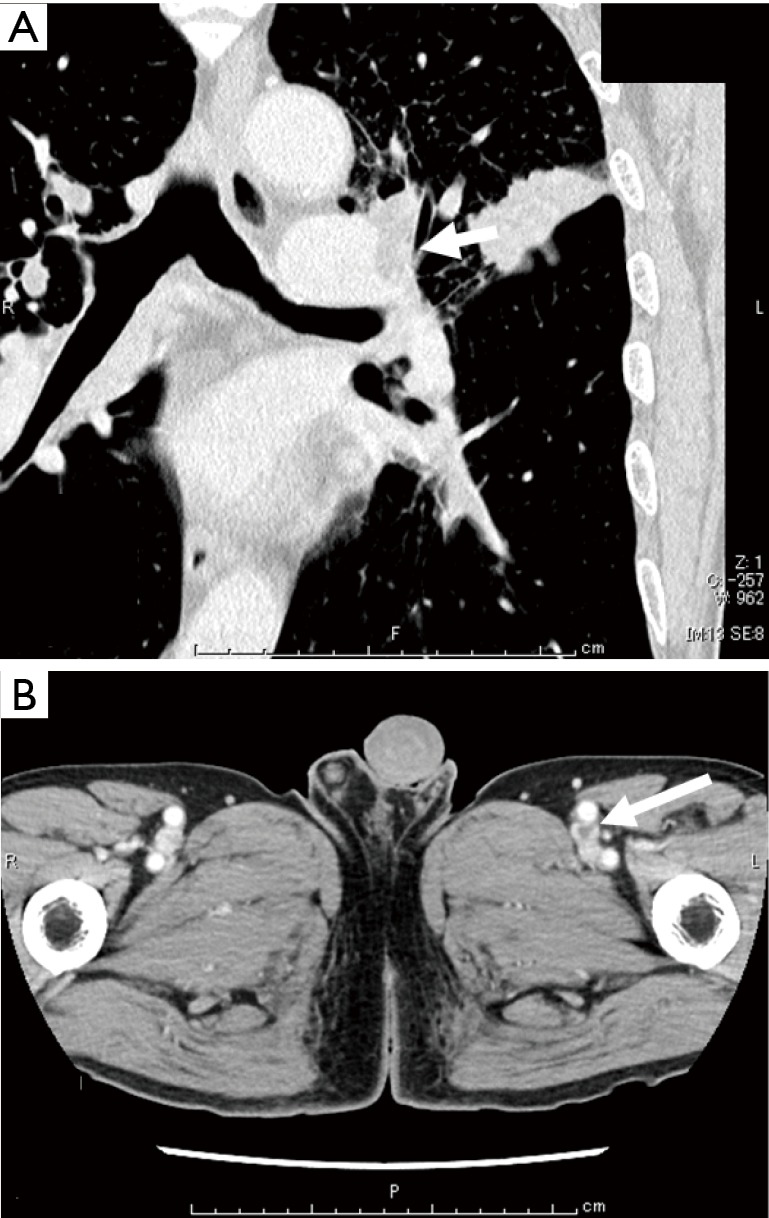
Whole-body contrast-enhanced CT. (A) Chest computed tomography (CT) revealed pulmonary artery thrombosis (see arrow). (B) Whole-body contrast-enhanced CT also revealed thrombosis of the left femoral vein (see arrow).
Further examination via leg ultrasound revealed multiple venous thromboses. His serum D-dimer level was high (10.8 µg/mL). No thrombophilia was observed, and levels of protein S, activated protein C, lupus anticoagulant, and anti-cardiolipin antibody were 130%, 168%, 1.18, and 5.0 U/mL, respectively. He was thus diagnosed with Trousseau’s syndrome and considered at high risk for surgery. Anticoagulant therapy was administered via venous infusion of unfractionated heparin, following which oral Xa inhibitor was administered. After confirming that his D-dimer level had decreased, we initiated chemotherapy with cisplatin (CDDP) and pemetrexed (PEM). During chemotherapy, we monitored clinical manifestations and serum D-dimer levels to prevent exacerbation of the thromboembolism. After four courses of CDDP+PEM therapy and one course of maintenance PEM therapy, the main tumor had shrunk, and his lymphangitis of the left upper lobe had resolved on chest CT (Figure 3). CT further revealed that his bilateral pulmonary artery and left femoral artery thromboses had resolved. While D-dimer levels had decreased to 0.5 µg/mL prior to the operation, his renal function had decreased to an estimated glomerular filtration rate (eGFR) of 42.0. Thus, we chose to perform salvage surgery rather than continuous PEM chemotherapy. Six months after initiating chemotherapy, the patient underwent left upper lobectomy and lymph node resection. The postoperative pathological diagnosis was invasive adenocarcinoma with N1 lymph node metastasis, and the effectiveness of chemotherapy was regarded as EF2, ypT3(PM1)pN1cM0 stage IIIA (Figure 4A,B).
Figure 3.
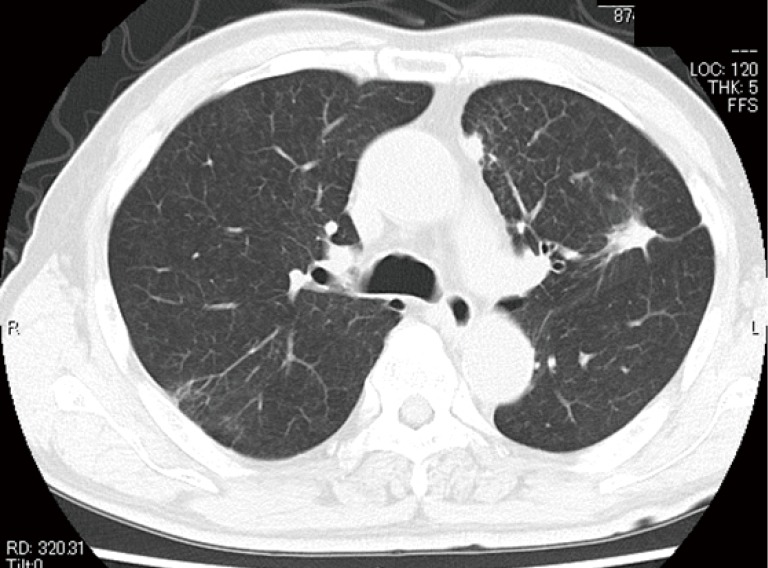
After four courses of CDDP+PEM therapy and one course of maintenance PEM therapy, the main tumor had shrunk, and lymphangitis of the left upper lobe had resolved on chest computed tomography (CT). CDDP, cisplatin; PEM, pemetrexed.
Figure 4.
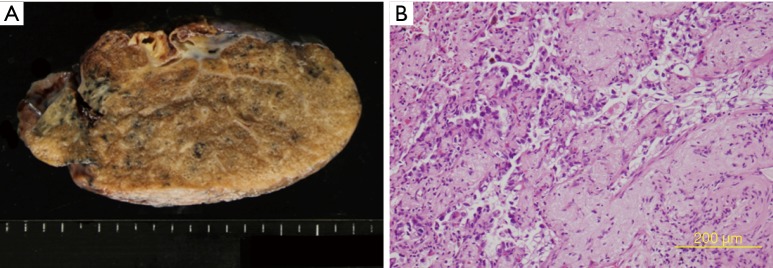
Pathological findings. (A) Macroscopic findings of the resected specimen. The tumor was 13 mm × 6 mm in size, and intrapulmonary metastasis was observed (H&E stain). (B) The postoperative pathological diagnosis was invasive adenocarcinoma with N1 lymph node metastasis. Chemotherapy effectiveness was rated as EF2, ypT3(PM1)pN1cM0 stage IIIA.
Discussion
Venous thromboembolism (VTE) is often associated with malignant tumors, representing the leading cause of death in patients with cancer. In 1865, Trousseau first reported the relationship between VTE features and malignancy (1), which is now referred to as paraneoplastic syndrome with solid malignancies. Trousseau’s syndrome is characterized by thromboembolism associated with malignancy, including nonbacterial thrombotic endocarditis (NTBE) and arterial thromboembolism (ATE). However, classic TS primarily involves VTE with or without arterial thrombosis in patients with malignancy who do not exhibit thrombophilia (5). Prognosis is generally poor among patients with solid cancers combined with VTE (3,4). Furthermore, prognosis among patients with lung cancer complicated by VTE is poor, with a median survival time (MST) of just 35–40 weeks (6,7).
Although the mechanisms underlying the development of coagulopathy in patients with malignancy remain unclear, previous studies have suggested that tissue factors, procoagulants, and monocytes/macrophages are activated by the tumor cells. Such activation in turn leads to the release of cytokines [i.e., interleukin (IL-1), IL-6, tumor necrosis factor], the production of additional tissue factors, and the activation of vascular endothelial cells. Other studies have further noted that carcinoma mucin molecules activate platelets (6,8).
In patients with solid cancer and VTE, both cancer treatment and anticoagulant therapy are necessary (9). Based on clinical stage (T3N0-1M0, stage IIB–IIIA), our patient’s lung cancer was deemed operable. However, given the increased risk of sudden death due to thrombosis in our patient, who exhibited multiple VTEs and pulmonary artery thrombosis, we were unable to perform surgery first. Thus, he was first treated with chemotherapy and anticoagulant therapy.
Low molecular weight or unfractionated heparin is recommended for patients with cancer-related VTE. Recent studies have also indicated that direct oral anticoagulants (DOACs) are safe and effective for the treatment of VTE in patients with cancer (10-13). In this case, we first administered unfractionated heparin, followed by an oral Xa inhibitor. We also monitored serial D-dimer levels, as these are reported as good markers of VTE in patients with cancer (14,15).
No consensus has been established regarding salvage surgery for lung cancer, and no previous reports have discussed cases of salvage surgery for lung cancer in patients with VTE. Nevertheless, we attempted salvage surgery in our patient due to his young age, early clinical cancer staging, and limited renal function. Given that his eGFR level had decreased substantially, we were unable to continue chemotherapy with PEM. Although other treatment options such as alternative chemotherapy or immune checkpoint inhibitor therapy were considered prior to surgery, we aimed to achieve complete resection via salvage surgery. Unfortunately, the final histological report for our patient indicated that the main tumor had shrunk to 13 mm, resulting in a chemotherapy effectiveness of EF2. In addition, metastasis to the hilum lymph nodes had occurred. Thus, the patient will require careful follow-up in the future.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Trousseau A. Phlegmasia alba dolens. Clinique Medicale de l’Hotel Dieu de Paris 1865;3:654-712. [Google Scholar]
- 2.Kanaji N, Watanabe N, Kita N, et al. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol 2014;5:197-223. 10.5306/wjco.v5.i3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846-50. 10.1056/NEJM200012213432504 [DOI] [PubMed] [Google Scholar]
- 4.Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003;107:I17-21. 10.1161/01.CIR.0000078466.72504.AC [DOI] [PubMed] [Google Scholar]
- 5.Mukai M. Thrombosis and treatment in patient with malignancy. Trousseau’s Syndrome. Thromb Med 2017;7:108-13. [Google Scholar]
- 6.Sugino K, Isobe K, Kikuchi N, et al. Clinical analysis of lung cancer associated with venous thromboembolism. Jpn J Lung Cancer 2009;49:151-6. 10.2482/haigan.49.151 [DOI] [Google Scholar]
- 7.Kanaji N, Mizoguchi H, Inoue T, et al. Clinical features of patients with lung cancer accompanied by thromboembolism or disseminated intravascular coagulation. Ther Clin Risk Manag 2018;14:1361-8. 10.2147/TCRM.S164700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood 2007;110:1723-9. 10.1182/blood-2006-10-053736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachihara M, Nikaido T, Wang X, et al. Four cases of Trousseau's syndrome associated with lung adenocarcinoma. Intern Med 2012;51:1099-102. 10.2169/internalmedicine.51.6453 [DOI] [PubMed] [Google Scholar]
- 10.Mandalà M, Falanga A, Roila F, et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guidelines for the management. Ann Oncol 2010;21 Suppl 5:v274-6. 10.1093/annonc/mdq199 [DOI] [PubMed] [Google Scholar]
- 11.Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: american society of clinical oncology clinical practice guideline update 2014. J Clin Oncol 2015;33:654-6. 10.1200/JCO.2014.59.7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vedovati MC, Germini F, Agnelli G, et al. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest 2015;147:475-83. 10.1378/chest.14-0402 [DOI] [PubMed] [Google Scholar]
- 13.Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res 2015;136:582-9. 10.1016/j.thromres.2015.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost 2017;117:219-30. 10.1160/TH16-08-0615 [DOI] [PubMed] [Google Scholar]
- 15.Ito S, Kikuchi K, Ueda A, et al. Changes in Serial D-Dimer Levels Predict the Prognoses of Trousseau's Syndrome Patients. Front Neurol 2018;9:528. 10.3389/fneur.2018.00528 [DOI] [PMC free article] [PubMed] [Google Scholar]