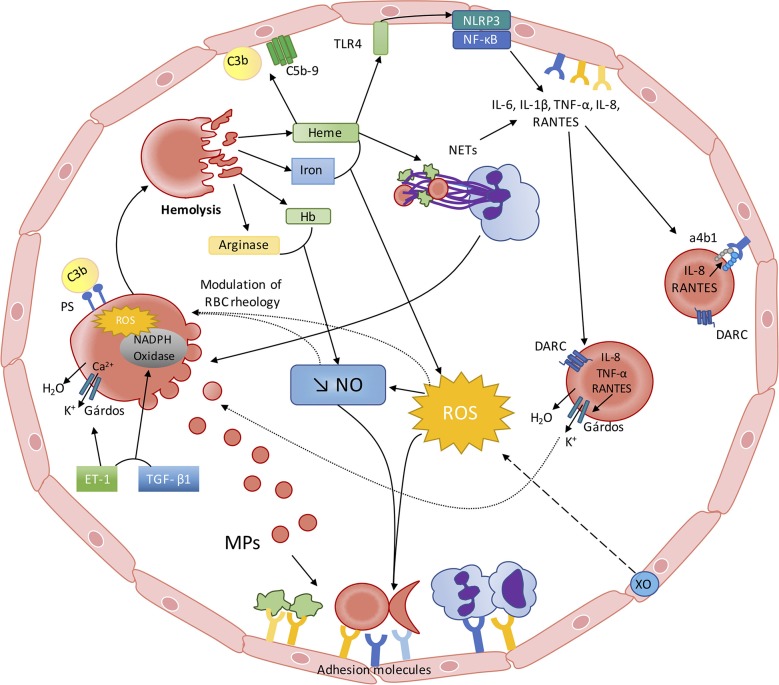
Figure 1.
The red blood cell—inflammation vicious circle in sickle cell disease. From RBC alterations to oxidative stress, inflammation and endothelial dysfunction: Sickled RBC are very fragile and prone to hemolyze. Hemolysis leads to the release of heme, iron, Hb and arginase into the plasma, which interfere with the metabolism/bioavailability of NO: (I) free arginase may hydrolyze the NO precursor Arginine; (II) free Hb scavenges NO at a rate of 1,000-fold faster than Hb encapsulated in the RBCs; (III) heme and iron increase ROS generation, which lead to the production of peroxynitrite. ROS production is also enhanced by Xanthine Oxidase activation, caused by the repetition of ischemic/reperfusion events. Decreased NO bioavailability and increased ROS activate endothelial cells, which in turn express adhesion molecules of both the CAM and Selectin families, promoting cell-cell interactions. Free heme is able to activate endothelial TLR4, which promotes inflammasome activation and cytokines production through NF-κB activation. Heme may also activate neutrophils, which would release NETs that can also affect endothelial cells and act as a scaffold for platelets and RBCs. Recent evidence also showed that free heme could stimulate the complement pathway with potential consequences at the endothelial cell level. From inflammation and oxidative stress to RBC alterations: This pro-inflammatory and pro-oxidative environment, resulting from sickle RBCs alterations, also impacts on RBC rheology and physiology. Increased ROS production may lower RBC deformability and increase RBC aggregation. Decreased NO bioavailability could also participate in the decrease of RBC deformability and promote eryptosis. NETs could also promote RBC eryptosis. Circulating inflammatory molecules, such as ET-1 and TGF-β, may activate RBC NADPH Oxidase, which in turn would produce ROS and further alter RBC. ROS and ET-1 are known to activate the RBC Gárdos channel, which could favor RBC dehydration and further promote HbS polymerization. The enhanced release of MP by sickled RBCs could further exacerbate inflammation and oxidative stress. Increased RBC phosphatidylserine exposure may favor the binding of complement proteins at the surface of RBCs, which can induce their lysis. RBCs also act as a reservoir and/or a sink for pro-inflammatory cytokines/chemokines. IL-8, TNF-α, and RANTES promote RBC dehydration through Gárdos channel activation in RBCs expressing DARC. IL-8 and RANTES can also lead to the activation of α4β1 integrin in sickle reticulocytes expressing DARC, contributing to the adhesion of these cells to the endothelium.