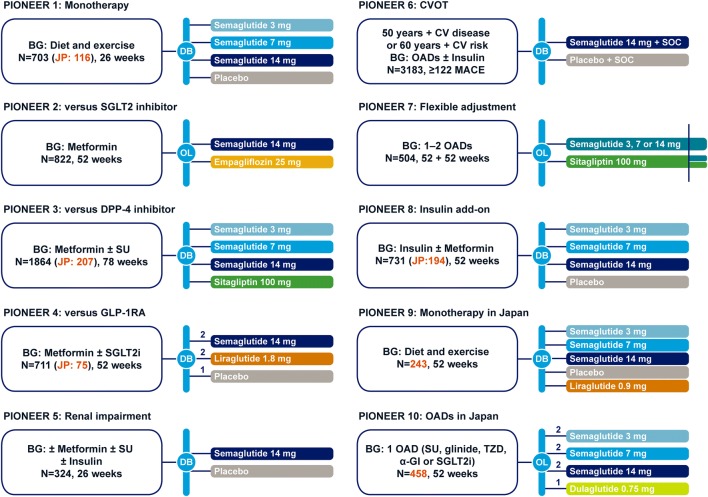
Fig. 2.
PIONEER clinical trial program. Time to primary endpoint: 26 weeks for PIONEER 1, 2, 3, 4, 5, and 8 trials. BG, background medication; CV, cardiovascular; CVOT, cardiovascular outcomes trial; DB, double-blind; DPP-4i, dipeptidyl peptidase-4 inhibitor; α-GI, α-glucosidase inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; JP, Japan; MACE, major adverse cardiovascular event; OAD, oral anti-diabetes drug; OL, open-label; PIONEER, peptide innovation for early diabetes treatment; SGLT2i, sodium− glucose cotransporter-2 inhibitor; SOC, standard of care; SU, sulfonylurea; TZD, thiazolidinedione