Abstract
Objectives
To improve visualization of suspicious lesions of the oral mucosa and to assess the accuracy of Velscope in assessing cellular changes occurring in oral premalignancy for early diagnosis.
Materials and methods
In this prospective, randomized in-vivo clinical study a total of 250 patients who gave history of chewing tobacco were screened. The selection of the site of biopsy was taken based on the area of loss of fluorescence identified by the Velscope within the lesion. Routine blood investigations were done. A biopsy was performed to confirm the findings of clinical examination. The data was collected and analysed.
Results
Among 200 patients only 110 underwent incisional biopsy. Of these only 89 patients showed neoplastic changes. Of the control biopsies, none of them showed any dysplastic changes. Out of 106 who exhibited speckling under autofluorescence, only 89 showed dysplastic changes whereas only 17 showed no dysplastic changes. Out of these 17 specimens, the histopathological diagnosis of 5 was coated tongue, 3 were pigmented lesions, 3 were geographic tongue and 2 were mucositis. Of the remaining 4, the histopathological diagnosis of 1 was oral submucous fibrosis, 1 was lichen planus and 2 were frictional keratosis.
Conclusion
False positive findings are possible in presence of highly inflamed tissues, and it is possible that use of Velscope alone may result in failure to detect regions of dysplasia, but it has its use definitely to improve clinical decision making about the nature of oral lesions and aids in decisions to biopsy regions of concern. Use of the scope has allowed practitioners to identify the best region for biopsy. It is much better to occasionally sample tissue that turns out to be benign than to fail to diagnose dysplastic or malignant lesions. However, poor specificity is a major limitation for using it as a screening tool.
Keywords: Oral premalignant lesions, Oral potentially malignant disorders, Velscope, Autofluorescence, Non-invasive diagnostic techniques
1. Introduction
The incidence of oral cancer worldwide, is approximately 3% of all malignancies and this is creating a remarkable health problem.1 Oral cancer is showing a rise in incidence by every passing year and is being recognized in the late stage. Late detection and diagnosis can be attributed to the inadequacy in training of health professionals. Early diagnosis of curable precursors of malignancy is still the best way to make sure that there is improvement in both survival rate and quality of life, along with the eminent advances in cancer treatment.2,3 Generally, the primarily important factor that decides the mortality rate in these patients is the clinical stage during diagnosis of the same. The diagnosis of oral cancer is influenced to a great extent by not only the patient reluctance to consult or due to inability to access a health care professional but also professional delay in diagnosis. The patients’ survival rate can be improved if the lesion before its malignant progression from leukoplakia is diagnosed at an early stage. The early detection and screening plays a major role in decreasing the morbidity and mortality of the disease.4,5
Early diagnosis of oral malignancy makes the treatment early and unaggressive and also boosts the survival rate to 80%.6 Oral health care professionals are the ones who detect malignancy and premalignant conditions in the early stages and they make a considerable contribution in decreasing the incidence and identifying the high risk patients and imparting good healthy habits education to them. By means of the combination of visual examination and palpation, detection of epithelial changes in oral mucosa is the main approach presently and is well known to be confined to an impressionistic interpretation. This is then followed by tissue biopsy with the histopathological assessment that is considered as the gold standard for the diagnosis,7 but this requires a well-trained health care professional and is also believed to be invasive, painful, expensive and time consuming. Any approach that makes easy the visualization of a dubious lesion could help a clinician to detect oral cancer in its early stages. Hence, the reason why there has been an evolution of several light induced fluorescence visualization appliances like the VELscope (Visually enhanced lesion scope), is due to the increased demand for non-invasive tests that will intensify the regular white light oral examination for the diagnosis of potentially malignant lesions. This system presents with a sensitivity of 98% and specificity of 96%–100%.8, 9, 10 Under a hypothesis, there could exist a molecular difference in the squamous cell carcinoma occurring in western and eastern populations. Accordingly there could be a difference in the behavior of these cells in other aspects also. Thus this study was designed to test the efficacy of VELscope in assessing the cellular changes occurring in oral premalignancy in Indian population. This study is laid on the usefulness of the Velscope as a diagnostic aid to assess its efficacy in distinguishing the cellular changes in oral premalignancy.
The working principle of the Velscope system relies on the loss of fluorescence in visible and non -visible high risk oral lesions can be identified by applying direct fluorescence. It comprises of a light source that emits a wavelength of 400–460 nm and a manual unit for visualization. Green auto-fluorescence is emitted by the normal mucosa whereas the fluorescent light is absorbed by abnormal areas making them appear dark, under this light[Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6]. Normally occurring fluorophores in the tissue are a basis for the mechanism of tissue fluorescence that determine their reflective and absorptive pattern. Different profile areas undergoing malignant changes in which loss of fluorescence visualization is seen may maximize on the exposure to blue light spectra (400–460 nm). Therefore, pre-malignant changes are diagnosed even before its clinical appearance. Thus the Velscope system can be used as a complement to visual examination for:
-
1.
Distinguishing between normal and abnormal tissues (both benign and malignant) is enhanced.
-
2.
Benign and neoplastic changes can be differentiated and/or delineated.
-
3.
Dysplastic or malignant lesions margins that are invisible to the naked eye under white light can be visualized.9
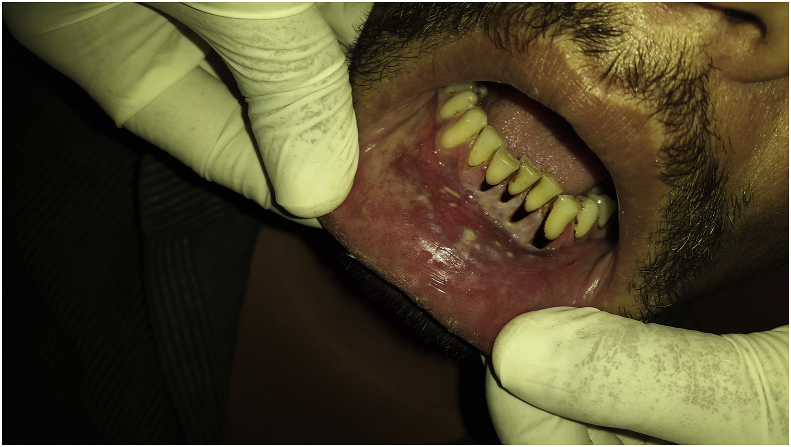
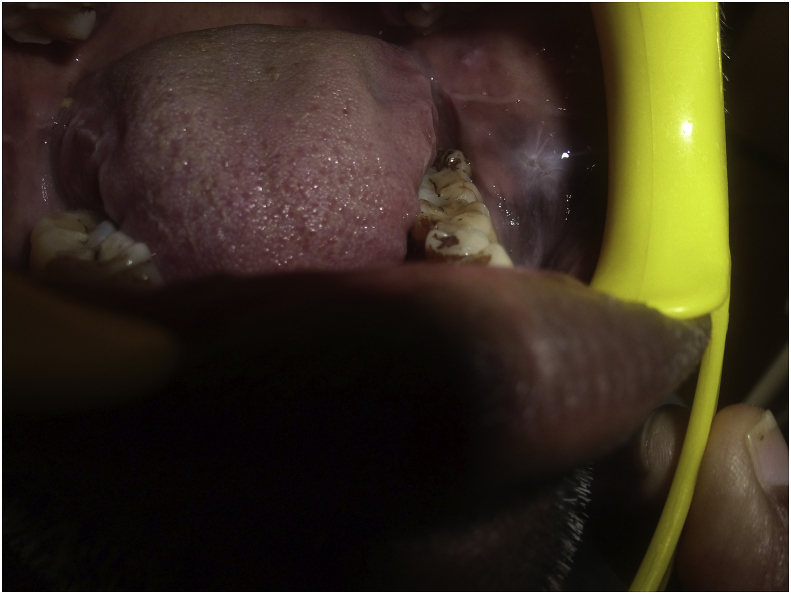
Fig. 1.
Lichen planus.
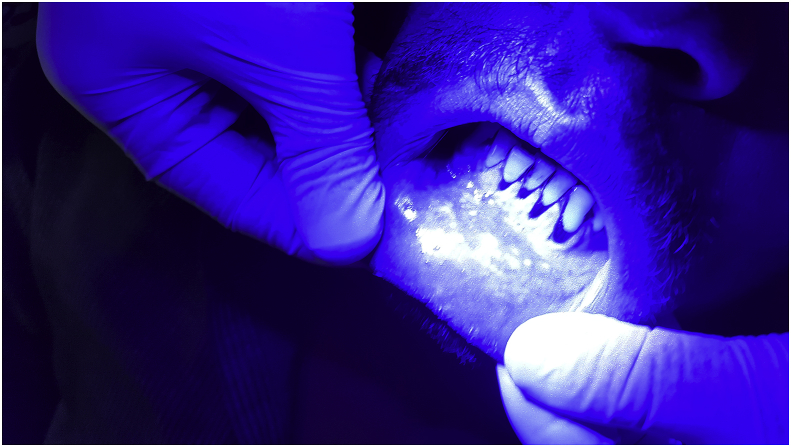
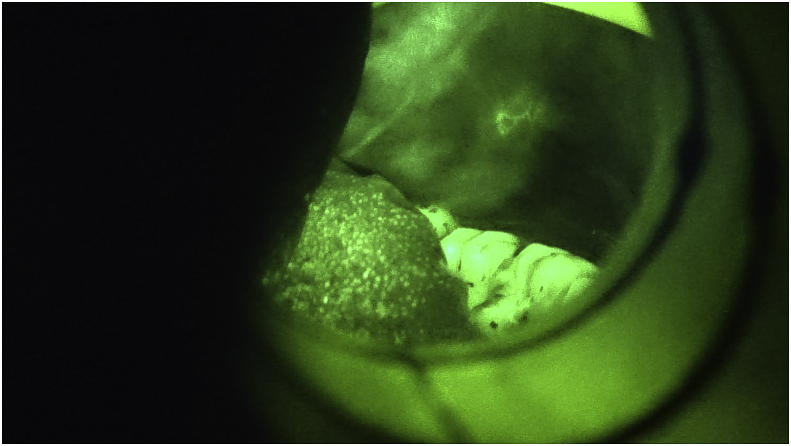
Fig. 2.
Lichen planus under blue light. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Leukoplakia.
Fig. 4.
Leukoplakia under blue light. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
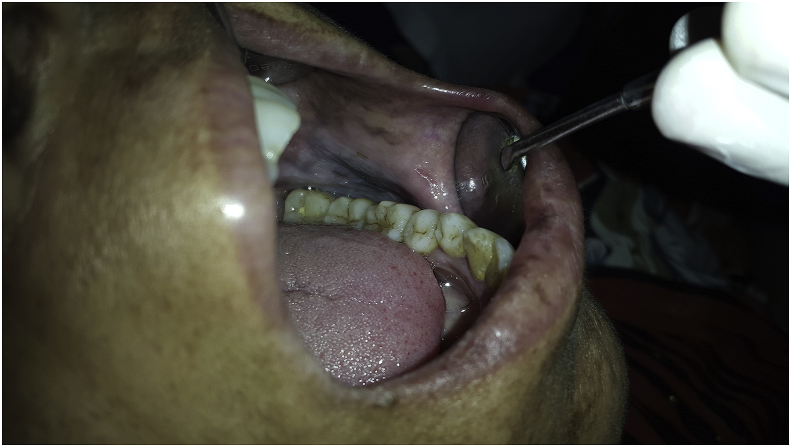
Fig. 5.
Linea alba.
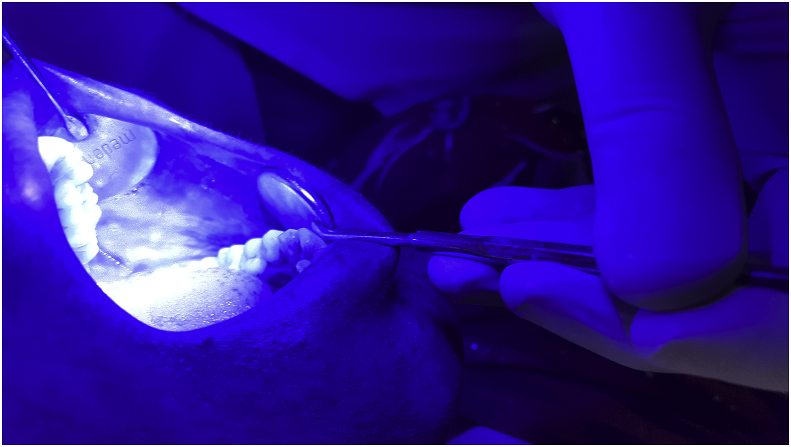
Fig. 6.
Linea alba under blue light. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. Materials and methods
2.1. Source of data
A prospective, randomized in-vivo clinical study was carried out in the Department of Oral and Maxillofacial Surgery after receiving approval from the ethics committee at Dr. D.Y.Patil Dental College and Hospital, Pimpri, Pune. [Institutional Review Board No. DPU/R&R(D)/159(8)2013].
2.2. Method of collection of data
A total of 250 patients who presented to our department with positive history of tobacco/pan/supari gutkha chewing and/or smoking habits were explained about the study and out of these only 200 consented for the research project. They were categorized according to age, sex, clinical diagnosis when examined in white light and assessing the cellular changes using Velscope which is the source of blue light (Table 1). Informed consent was taken before the procedure. The selection of the site of biopsy was taken based on the area of loss of fluorescence identified by the Velscope within the lesion. Routine blood investigations were done and a biopsy was performed to confirm the findings of clinical examination. All the patients were put on a short course of antibiotics and analgesics after the biopsy. Biopsy was not done for patients who did not have any clinically visible lesion and also did not show loss of fluorescence and these patients were re-assured that no cancerous change was evidently seen in the mouth. However to obtain the unbiased accurate estimates of the sensitivity and specificity we scrutinized the control biopsies as a subsample of the areas that looked non-dubious in 5 patients. The data was collected and distinctions and coalition between the autofluorescence tests and biopsy reports were examined. The patients were counseled about stoppage of the tobacco chewing habit and were followed up for the next three years.
Table 1.
Disease specific profile of patients. (n-110).
| No. of patients | Gender | Mean Age in years | Clinical Diagnosis |
|---|---|---|---|
| 35 | M- 30 F-5 | 43 | Leukoplakia |
| 16 | M- 14 F-2 | 38 | Lichen planus |
| 12 | M- 11 F-1 | 52 | Oral submucous fibrosis |
| 4 | M- 2 F-2 | 59 | Candidosis |
| 8 | M- 08 | 30 | Frictional keratosis |
| 2 | M- 02 | 29 | Smokers palate |
| 5 | M- 05 | 46 | Coated tongue |
| 20 | M- 19 F-1 | 44.5 | Erythroplakia |
| 2 | M- 02 | 62 | Mucositis |
| 3 | M- 03 | 64 | Pigmented areas |
| 3 | M- 03 | 54 | Geographic tongue |
2.3. Materials used for data collection
-
➢
Velscope and the accessories
-
➢
Local anesthetic solution
-
➢
5 cc disposable syringe
-
➢
1 inch needle, 25 gauge
-
➢
BP handle and no. 15 blade
-
➢
Adson's tooth forceps
-
➢
Biopsy bottle containing formalin
-
➢
Needle holder
-
➢
3-0 black braided silk
-
➢
Curved needle
-
➢
Suture cutting scissors
3. Results
A total of 200 patients, 175 males and 25 females (age range being between 25 yrs and 65 yrs) who presented to our department with positive history of tobacco chewing and/or smoking habits were enrolled for this study. After a complete visual and autofluorescence examination, all those patients who showed either clinically visible oral lesions and/or lack of autofluorescence (n = 113, males- 99 and females- 14) were advised biopsy. However only 110 underwent incisional biopsy for histopathological assessment. 3 patients did not agree for biopsy due to fear of the procedure and socio-economic problems. Of these 110 patients, only 89 patients showed histopathological presence of neoplastic changes like dysplasia, carcinoma in situ and squamous cell carcinoma(Table 2). Of the control biopsies, none of them showed any signs of dysplastic changes.
Table 2.
Comparison of autofluorescence results and histopathological assessment.
| Diagnosis | No.of patients | Examination under velscope | Histopathological assessment | sensitivity | Specificity |
|---|---|---|---|---|---|
| Leukoplakia | 35 | All + ve | Neoplastic changes evident | 100% | – |
| Lichen planus | 16 | 14 +ve 2 -ve |
13 - mild dysplastic changes evident 3- no dysplastic change |
81% | 18% |
| Oral submucous fibrosis | 12 | 11 +ve 1 -ve |
10- mild dysplastic changes evident 2- no dysplastic change |
83% | 16% |
| Candidosis | 4 | 3 +ve 1 -ve |
3- minimal dysplastic changes evident 1- inflammatory changes seen. |
75% | 25% |
| Frictional keratosis | 8 | All + ve | 6 -carcinoma in situ 2- benign tissue |
75% | 25% |
| Smokers palate | 2 | All + ve | Carcinoma in situ | 100% | – |
| Coated tongue | 5 | All + ve | No dysplastic change | 0% | – |
| Erythroplakia | 20 | All + ve | Mild to moderate dysplastic changes evident | 100% | – |
| Mucositis | 2 | All + ve | No dysplastic change | 0% | – |
| Pigmented areas | 3 | All + ve | No dysplastic change | 0% | – |
| Geographic tongue | 3 | All + ve | No dysplastic change | 0% | – |
We found that out of 106 who exhibited speckling under autofluorescence, only 89 showed dysplastic changes on histopathological examination whereas only 17 showed no dysplastic changes. Out of these 17 specimens, the histopathological diagnosis of 5 was coated tongue, 3 were pigmented lesions, 3 were geographic tongue and 2 were mucositis. Of the remaining 4, the histopathological diagnosis of 1 was oral submucous fibrosis, 1 was lichen planus and 2 was frictional keratosis. These were clinically visible lesions under white light examination (Table 2).
4. Discussion
The overall 5 year survival rate for oral cancer has remained low at approximately 50% for the past decade.10 This in part, accounts to the failure in the early diagnosis of potentially malignant disorders either due to patient ignorance or lack of access of medical services. Thus, there is a strong need to improve the diagnostic approaches of the primary health care professional and the maxillofacial surgeons, also providing less interventional investigations. This area remains an important part of research agenda. As per primitive research, normal from malignant tissue can be differentiated using a system that works on optical diagnostics where-in light-tissue interactions can be assessed. Light based detection systems have been developed to identify tissue changes which occur in malignancy. One of such optical mechanization is called autofluorescence which is available in the market by the brand name “Velscope”.1,7, 8, 9,11 It is a handy device which percieves alterations in normal fluorescence (apple green hue) which is analogous with morphological and biochemical changes during cancer development producing a dark shadow on autofluorescence. Among the many factors that influence tissue autofluorescence, are tissue construction, light absorption and dispersion properties of each layer of tissue, the dispensation and congregation of fluorophores in the different tissue layers, the surrounding environment of the tissue, and its metabolic status, the differentiation from benign to neoplastic tissue is attributed to the tissue autofluorescence patterns that reflect changes in tissue composition. This in itself is a complex phenomenon.12
Velscope is simple to use and noninvasive. It can be utilized by a vast range of clinicians after a precise duration training and has limited operator variability. It has no recurrent cost since no consumable reagents are needed. It provides real time results and involves less time consuming procedure. It has a high sensitivity for any oral mucosal disorder. However it has a few limitations like high initial setting-up cost. It requires a dark environment and no permanent record is maintained unless photographed (compatible camera is available) and also has a low specificity for dysplasia. However, the simplicity of use, extensive area imaging potentiality, non-requirement of estranged means and the capability to note dispersed lesions impart autofluorescence an effective benefit as a superintendence apparatus.
The use of autofluorescence screening in vivo for oral cancer dates back to the 1980s.12 Early studies have demonstrated inconsistent results, but the studies conducted in the recent years have reported results which prove a significant improvement over white light examination.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Only a few oral lesions whereas predominantly laryngeal lesions were assessed in these studies. It was evaluated the effect of autofluoresence of four different wavelengths on freshly resected oral tissue and concluded that the supreme contrast was achieved at the wavelength of 400 nm.16 Then a handy autofluorescence appliance was used and successfully displayed the capability to identify new lesions and enlarged tumor margins that were imperceptible on white light examination.17
In our study, all our patients who showed speckling on autofluorescence, were under taken for biopsy and we found that out of 106 who exhibited speckling under autofluorescence, 89 showed dysplastic changes on histopathological examination whereas 17 showed no dysplastic changes. Out of these 17 specimens, the histopathological diagnosis of 5 was coated tongue, 3 were pigmented lesions, 3 were geographic tongue and 2 were mucositis. Of the remaining 4, the histopathological diagnosis of 1 was oral submucous fibrosis, 1 was lichen planus and 2 was frictional keratosis. These were clinically visible lesions under white light examination. The sensitivity and specificity was calculated (Table 2).
Formulas for calculating sensitivity and specificity values of autofluorescence8
| Sensitivity = No. of true positives/ No. of true positives + No. of false negatives |
and
| Specificity = No. of true negatives/ No. of true negatives + No. of false positives |
Being watchful while surveying the results of autofluorescence screening studies does matter to a great extent, even though total sensitivity and specificity for constancy purposes were outlined. Preferably, the needfulness of the precise gauges of the “true negatives” and the “false negatives” is needed to acquire the unprejudiced rate of the sensitivity and specificity. These can be acquired only by histopathologic diagnosis from all the usual areas under white light examination and autofluorescence. In this study, since taking biopsies from the whole oral cavity was not a realistic alternative, the control biopsies were surveyed as a subsample of the non-suspicious areas under white light examination as well as autofluorescence, in 5 patients. Signs of dysplastic changes weren't seen in any of these control biopsy specimens. Our results show that Velscope has a high sensitivity towards dysplastic and neoplastic changes but indigent specificity is a crucial constraint for using this device as a screening tool in a paramount care setup.
5. Conclusion
To prevent the progression of oral precancerous lesions and disorders to later stages requires sound knowledge and education to detect oral cancer at early stages. Hence it is imperative to increase the health care providers’ depth of knowledge of disease and the latest technology of non-invasive detection tools available for early detection of this devastating disease to improve patient prognosis and survival.
False positive findings are possible in presence of highly inflamed tissues, and it is possible that use of Velscope alone may result in failure to detect regions of dysplasia, but it has its use definitely to improve clinical decision making about the nature of oral lesions and aids in decisions to biopsy regions of concern. Where tissue changes are generalized or cover significant areas of the mouth, use of the scope has allowed practitioners to identify the best region for biopsy. It is much better to occasionally sample tissue that turns out to be benign than to fail to diagnose dysplastic or malignant lesions. However, poor specificity is a major limitation for using autofluorescence as a screening tool in a primary care setting.
Funding
Dr. D.Y. Patil Vidyapeeth, Pimpri, Pune.
Declaration of competing interest
None.
Acknowledgement
None.
Contributor Information
Sonal Shah, Email: sonalbshah@rediffmail.com.
Pushkar Waknis, Email: pushkarwaknis@gmail.com.
Aditi Saha, Email: dr.aditisaha@gmail.com.
Sneha Setiya, Email: setiya_sneha@yahoo.com.
Tusha Ratra, Email: tusharatra@gmail.com.
References
- 1.Kharma M.Y., Alalwani M.S., Amer M.F. Promising future in the detection of oral cancer by using advance screening technology. J Oral Health Craniofac Sci. 2016;1 022-33. [Google Scholar]
- 2.Messadi D.V. Diagnostic aids for detection of oral precancerous conditions. Int J Oral Sci. 2013;5(2):59. doi: 10.1038/ijos.2013.24. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigneswaran N., Koh S., Gillenwater A. Incidental detection of an occult oral malignancy with autofluorescence imaging: a case report. Head Neck Oncol. 2009;1(1):37. doi: 10.1186/1758-3284-1-37. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson A.T., Corcuera M.M., Trapero J.C., Sánchez J.C., Martínez A.B. Analysis of new diagnostic methods in suspicious lesions of the oral mucosa. Medicina oral, patología oral y cirugía bucal. Ed. inglesa. 2009;14(5):1. [PubMed] [Google Scholar]
- 5.Fedele S. Diagnostic aids in the screening of oral cancer. Head Neck Oncol. 2009;1(1):5. doi: 10.1186/1758-3284-1-5. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo X., Xu H., He M. Accuracy of autofluorescence in diagnosing oral squamous cell carcinoma and oral potentially malignant disorders: a comparative study with aero-digestive lesions. Sci Rep. 2016;6 doi: 10.1038/srep29943. Jul 15. 29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rashid A., Warnakulasuriya S. The use of light‐based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med. 2015;44(5):307–328. doi: 10.1111/jop.12218. May. [DOI] [PubMed] [Google Scholar]
- 8.Khan U., Aziz A., Ahmed M. Autofluorescence/A clinical trial: a new hope for early detection of oral cancer and oral potentially malignant disorders. Pakistan Oral Dent. J. 2014;34(2) Jun 1. [Google Scholar]
- 9.Bhatia N., Matias M.A., Farah C.S. Assessment of a decision making protocol to improve the efficacy of VELscope™ in general dental practice: a prospective evaluation. Oral Oncol. 2014;50(10):1012–1019. doi: 10.1016/j.oraloncology.2014.07.002. Oct 1. [DOI] [PubMed] [Google Scholar]
- 10.Venugopal C., Nazeer S.S., Balan A., Jayasree R.S. Autofluorescence spectroscopy augmented by multivariate analysis as a potential noninvasive tool for early diagnosis of oral cavity disorders. Photomed. Laser Surg. 2013;31(12):605–612. doi: 10.1089/pho.2013.3547. Dec 1. [DOI] [PubMed] [Google Scholar]
- 11.Laronde D.M., Williams P.M., Hislop T.G. Influence of fluorescence on screening decisions for oral mucosal lesions in community dental practices. J Oral Pathol Med. 2014;43(1):7–13. doi: 10.1111/jop.12090. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein J.B., Güneri P., Boyacioglu H., Abt E. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J. Am. Dent. Assoc. 2012;143(12):1332–1342. doi: 10.14219/jada.archive.2012.0096. Dec 1. [DOI] [PubMed] [Google Scholar]
- 13.Reichart P.A. Oral mucosal lesions in a representative cross‐sectional study of aging Germans. Community Dent Oral Epidemiol. 2000;28(5):390–398. doi: 10.1034/j.1600-0528.2000.028005390.x. Oct. [DOI] [PubMed] [Google Scholar]
- 14.Thomson P.J. Field change and oral cancer: new evidence for widespread carcinogenesis? Int J Oral Maxillofac Surg. 2002;31(3):262–266. doi: 10.1054/ijom.2002.0220. Jun 1. [DOI] [PubMed] [Google Scholar]
- 15.Wax A., Yang C., Müller M.G. In situ detection of neoplastic transformation and chemopreventive effects in rat esophagus epithelium using angle-resolved low-coherence interferometry. Canc Res. 2003;63(13):3556–3559. Jul 1. [PubMed] [Google Scholar]
- 16.Svistun E., Alizadeh‐Naderi R., El‐Naggar A., Jacob R., Gillenwater A., Richards‐Kortum R. Vision enhancement system for detection of oral cavity neoplasia based on autofluorescence. Head Neck: J. Sci. Spec. Head Neck. 2004;26(3):205–215. doi: 10.1002/hed.10381. Mar. [DOI] [PubMed] [Google Scholar]
- 17.Poh C.F., Zhang L., Anderson D.W. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Canc Res. 2006;12(22):6716–6722. doi: 10.1158/1078-0432.CCR-06-1317. Nov 15. [DOI] [PubMed] [Google Scholar]
- 18.Balevi B. Evidence-based decision making: should the general dentist adopt the use of the VELscope for routine screening for oral cancer? J. Can. Dent. Assoc. 2007;73(7):603. Sep. [PubMed] [Google Scholar]
- 19.Patton L.L., Epstein J.B., Kerr A.R. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J. Am. Dent. Assoc. 2008;139(7):896–905. doi: 10.14219/jada.archive.2008.0276. Jul 1. [DOI] [PubMed] [Google Scholar]
- 20.Pavlova I., Williams M., El-Naggar A., Richards-Kortum R., Gillenwater A. Understanding the biological basis of autofluorescence imaging for oral cancer detection: high-resolution fluorescence microscopy in viable tissue. Clin Canc Res. 2008;14(8):2396–2404. doi: 10.1158/1078-0432.CCR-07-1609. Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roblyer D., Kurachi C., Stepanek V. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Canc Prev Res. 2009;2(5):423–431. doi: 10.1158/1940-6207.CAPR-08-0229. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayaprakash V., Sullivan M., Merzianu M. Autofluorescence-guided surveillance for oral cancer. Canc Prev Res. 2009;2(11):966–974. doi: 10.1158/1940-6207.CAPR-09-0062. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huff K., Stark P.C., Solomon L.W. Sensitivity of direct tissue fluorescence visualization in screening for oral premalignant lesions in general practice. Gen Dent. 2009;57(1):34–38. Jan. [PubMed] [Google Scholar]
- 24.Koch F.P., Kaemmerer P.W., Biesterfeld S., Kunkel M., Wagner W. Effectiveness of autofluorescence to identify suspicious oral lesions—a prospective, blinded clinical trial. Clin Oral Invest. 2011;15(6):975–982. doi: 10.1007/s00784-010-0455-1. Dec 1. [DOI] [PubMed] [Google Scholar]
- 25.Balevi B. Assessing the usefulness of three adjunctive diagnostic devices for oral cancer screening: a probabilistic approach. Community Dent Oral Epidemiol. 2011;39(2):171–176. doi: 10.1111/j.1600-0528.2010.00579.x. Apr. [DOI] [PubMed] [Google Scholar]
- 26.Pierce M.C., Schwarz R.A., Bhattar V.S. Accuracy of in vivo multimodal optical imaging for detection of oral neoplasia. Canc Prev Res. 2012;5(6):801–809. doi: 10.1158/1940-6207.CAPR-11-0555. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takano J.H., Yakushiji T., Kamiyama I. Detecting early oral cancer: narrowband imaging system observation of the oral mucosa microvasculature. Int J Oral Maxillofac Surg. 2010;39(3):208–213. doi: 10.1016/j.ijom.2010.01.007. Mar 1. [DOI] [PubMed] [Google Scholar]