Abstract
Pembrolizumab is an immune checkpoint inhibitor (ICI) that targets the programmed cell death (PD)-1 receptor. It significantly increases the overall survival in patients with locally advanced or metastatic urothelial cancer. However, its administration may induce serious immune-related adverse effects, such as myocarditis and myasthenia gravis (MG). Therefore, ICI treatment may have been withheld for MG patients with cancer. We report the first case in which pembrolizumab was used safely without aggravation of MG symptoms in right renal pelvic and bladder cancers, even though the anti-acetylcholine receptor antibody (anti-ACh-R Ab) serum concentration increased. The patient was a 70-year-old man diagnosed with stage III renal pelvic cancer and bladder cancer, with multiple liver metastases. He was previously diagnosed with MG at the age of 58 years. During second-line treatment with pembrolizumab, his anti-AChR Ab levels increased from 0.8 to 10.9, without exacerbation of MG symptoms. The liver metastases disappeared after five courses of pembrolizumab. This report shows that MG is not a reason to refrain from using PD-1 inhibitors in cancer patients; it should be considered when treatment is performed in highly experienced centers.
Keywords: Myasthenia gravis, Pembrolizumab, Metastatic urothelial cancer, Immune checkpoint inhibitor, Serum anti-acetylcholine receptor antibody
Highlights
-
•
Metastatic urothelial cancer with myasthenia gravis was treated with pembrolizumab.
-
•
Pembrolizumab increased the level of serum anti-acetylcholine receptor antibody.
-
•
Pembrolizumab therapy did not adversely affect the symptoms of myasthenia gravis.
-
•
Immune checkpoint inhibitors can be used in cancer patients with myasthenia gravis.
1. Case report
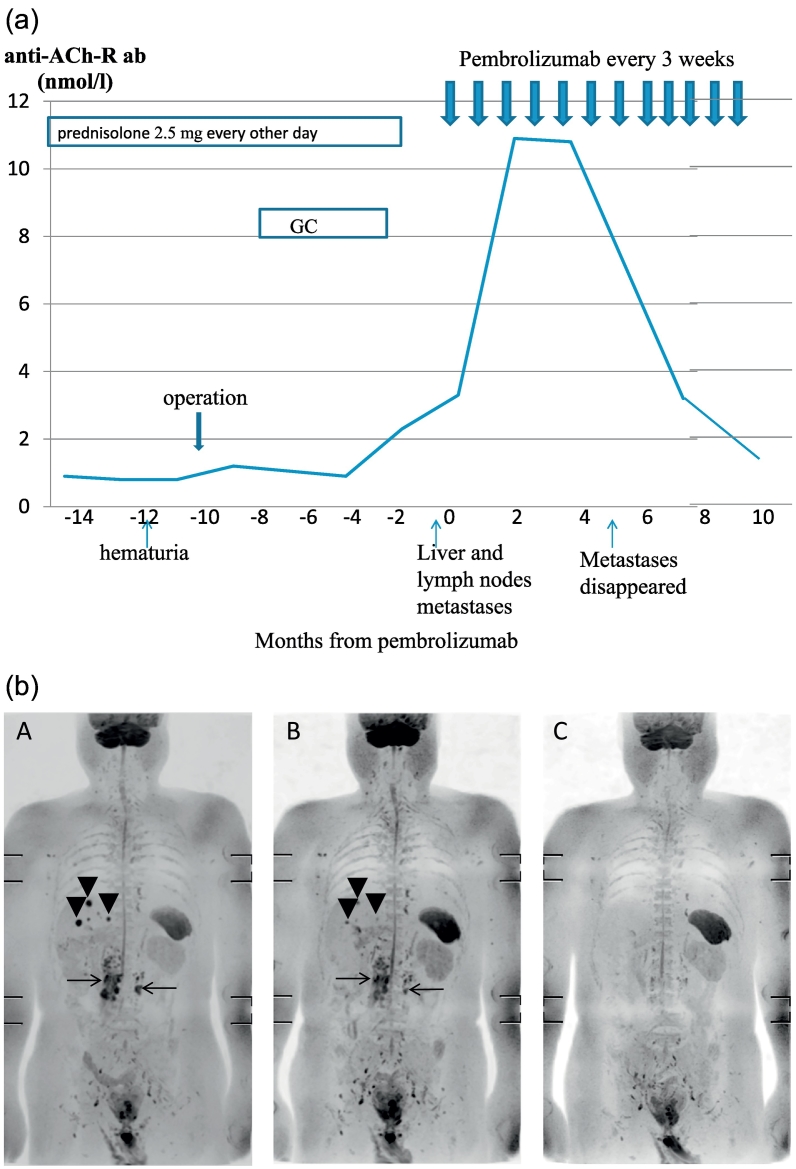
The patient was a 70-year-old man. At 58 years of age, he noticed right-eye ptosis and visited our Neurology department. The ptosis was characterized by diurnal variation, and he could not finish a nine-hole course of golf due to fatigue. Positive results on the Tensilon test, attenuations in the repetitive nerve stimulation test and an elevated concentration of anti-acetylcholine receptor antibody (anti-ACh-R Ab; 2.2 nmol/l; reference range, <0.2 nmol/l) informed a diagnosis of generalized MG. The patient was prescribed oral prednisolone and underwent thymectomy. Histology revealed involution of the thymus. His symptoms subsequently improved and were well-controlled with prednisolone taken at a dosage of 2.5 mg every other day. His anti-ACh-R Ab concentrations remained at 0.6–0.9 nmol/l. At 69 years of age, he visited the urology department because of hematuria. Abdominal computed tomography of the right renal pelvic and bladder confirmed the diagnosis of right renal pelvic cancer (T3N0M0) and bladder cancer (T1N0M0). Transurethral resection of the bladder tumor and minimum incision endoscopic right nephroureterectomy were performed. Adjuvant gemcitabine and cisplatin treatment were administered because of the adverse histological features of the high-grade urothelial carcinoma and invasion into the renal parenchyma. Multiple liver and retroperitoneal lymph node metastases were found 4 months after the treatment (Fig. 1a,b-A). Pembrolizumab treatment was subsequently planned as a second-line systemic therapy. The patient was instructed to stop taking steroids and felt transient fatigue and ptosis for approximately 1 week. His anti-ACh-R Ab levels rose to 10.9 nmol/l after three cycles of pembrolizumab (Fig. 1a). Despite the elevation of the antibody concentration, there was no exacerbation of MG symptoms were observed. The patient was able to play an 18-hole round of golf without fatigue. The anti-ACh-R Ab concentration was gradually decreased to 7.0 nmol/l after cycle 8 and to 3.2 mol/l after cycle 10 (Fig. 1a). Shrinkage of the liver and retroperitoneal lymph node metastases were observed after two cycles of pembrolizumab (Fig. 1b-B), and complete recovery was achieved after cycle 5 (Fig. 1b-C). Pembrolizumab treatment has been maintained for 8 months from its initiation without any adverse events except for the transient, asymptomatic elevation of anti-ACh-R Ab concentrations. The patient worked as a businessman and still enjoys playing golf occasionally.
Fig. 1.
a Clinical course of the patient.
The concentration of the anti-acetylcholine receptor antibody (anti-ACh-R Ab) was 3.4 nmol/l before pembrolizumab treatment began, increased to 10.9 nmol/l after three cycles of pembrolizumab, slightly reduced to 10.8 nmol/l after five cycles, and decreased to 7.0 nmol/l after the eight cycles. Liver metastases disappeared after five cycles of pembrolizumab. After 10 cycles, the anti-Ach-R Ab concentration was decreased to 3.2 nmol/l. After 12 cycles, the anti-Ach-R Ab concentration was decreased to 1.8 nmol/l. The Y-axis shows the anti-ACh-R Ab concentration, whereas the X-axis shows the months from when pembrolizumab treatment began.
GC: gemcitabine and cisplatin treatment.
b MRI (diffusion weighted images).
A. Before pembrolizumab treatment.
Multiple liver (arrowheads) and retroperitoneal lymph node metastases (arrows) are seen.
B. Two cycles after pembrolizumab treatment.
Size of liver (arrowheads) and retroperitoneal lymph node metastases (arrows) is decreased.
C. Five cycles after pembrolizumab treatment.
Liver and retroperitoneal lymph node metastases have disappeared.
2. Discussion
Overexpression of programmed cell death protein (PD)-1 is associated with favorable outcomes in cases of autoimmune diseases. Hence, it is feasible that PD-1 inhibition could result in the exacerbation of symptoms in patients with pre-existing MG [[1], [2], [3], [4], [5]].
Four previous reports have reported the use of pembrolizumab for treating patients with previously diagnosed MG [[2], [3], [4], [5]]. As each of the four patients had malignant melanoma [[2], [3], [4], [5]], this report is the first to document the administration of pembrolizumab to a patient with urothelial cancer and MG. Similar to our case, MG was in remission in all four cases [[2], [3], [4], [5]]. Pembrolizumab treatment was started between 4 and 29 years after the onset of MG [[2], [3], [4], [5]]. In three cases, immunosuppressants were reduced before pembrolizumab treatment commenced [[2], [3], [4]]. Two out of the four patients died [2,3]; both patients had dysphagia at the onset of MG [2,3].
No prior case reports resemble the present case; despite an elevated concentration of anti-ACh-R Ab, the patient presented no symptoms and required no immunotherapy. However, a similar response to nivolumab treatment has previously been reported; elevation of anti-ACh-R Ab concentrations was observed in an anti-ACh-R Ab-positive patient with cancer and an MG patient with post-operated thymic hyperplasia, but there were no symptoms [6,7].
It is impossible to identify patients whose MG does not deteriorate after treatment with an ICI. However, our case highlights the fact that the treatment may be feasible in some MG patients, and should be considered in highly experienced centers. No bulbar symptoms at the onset of MG may indicate that ICI can be safely administered. The effects of thymectomy need to be examined by a review of multiple cases.
Undoubtedly, even with good control of MG, careful observation by a neurologist remains necessary when prescribing pembrolizumab to patients with MG. Thus, even if patients have a history of MG, the opportunity of using pembrolizumab to treat cancer should not be rejected.
Informed consent
We obtained written informed consent from the patient to use his information, including case details, in this article.
Funding
This work was supported, in part, by a grant from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (Grant no. 16k07081).
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Johansen A., Christensen S.J., Scheie D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: systematic review. Neurology. 2019;92:663–674. doi: 10.1212/WNL.0000000000007235. [DOI] [PubMed] [Google Scholar]
- 2.Phadke S.D., Ghabour R., Swick B.L. Pembrolizumab therapy triggering an exacerbation of preexisting autoimmune disease: a report of 2 patient cases. J. Investig. Med. High Impact Case Rep. 2016;4 doi: 10.1177/2324709616674316. (2324709616674316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earl D.E., Loochtan A.I., Bedlack R.S. Refractory myasthenia gravis exacerbation triggered by pembrolizumab. Muscle Nerve. 2018;57:E120–E121. doi: 10.1002/mus.26021. [DOI] [PubMed] [Google Scholar]
- 4.KHV Lau, Kumar A., Yang I.H. Exacerbation of myasthenia gravis in a patient with melanoma treated with pembrolizumab. Muscle Nerve. 2016;54:157–161. doi: 10.1002/mus.25141. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J., Li Y. Myasthenia gravis exacerbation associated with pembrolizumab. Muscle Nerve. 2016;54:506–507. doi: 10.1002/mus.25055. [DOI] [PubMed] [Google Scholar]
- 6.Saruwatari K., Sato R., Naklane S. The risks and benefits of immune checkpoint blockade in anti-AchR antibody-seropositive non-small cell lung cancer patients. Cancers. 2019;11:140. doi: 10.3390/cancers11020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan B., Zierz S., Weber T., Jordan K. Successful use of an immune checkpoint inhibitor in a patient with myasthenia gravis in remission. Muscle Nerve. 2019;60(1):E7–E8. doi: 10.1002/mus.26495. [DOI] [PubMed] [Google Scholar]