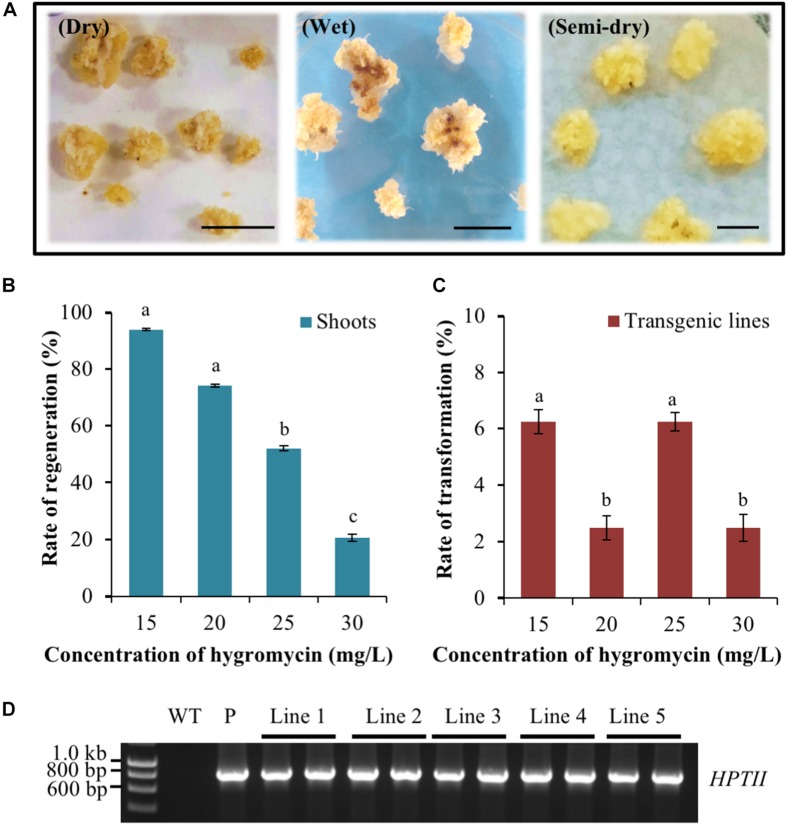
FIGURE 2.
Assessment of the Agrobacterium co-cultivation approach on Setaria viridis transformation efficiency and the use of hygromycin for in planta transformation selection. (A) Dry co-cultivation involved incubation of transfected calli on sterile filter paper discs. Wet co-cultivation involved incubation of calli with Agrobacterium on CIMC medium and additional washing steps to remove excess Agrobacterium. Semi-dry co-cultivation involved incubation of transfected calli on CIMC medium overlaid with filter paper discs. S. viridis calli showed the highest rate of recovery and optimal growth characteristics using the semi-dry co-cultivation approach. Bar = 0.5 cm. (B,C) The rate of shoot regeneration was the highest at 15 mg/L hygromycin, 92%, but this rate dropped substantially to 20% when the growth medium was supplemented with 30 mg/L hygromycin. Media supplemented with 25 mg/L hygromycin showed the most efficient reduction of non-transformant events while additionally allowing for the obtainment of the highest rates of genuine transformation events, at 6.3%. The results of statistical analysis are presented as letters on the histograms. The same letter indicates a non-statistically significant difference (p > 0.05), whereas a different letter indicates a statistically significant difference (p < 0.05). (D) PCR amplification analysis confirmed the presence of the pANIC12A selectable marker gene, HYGROMYCIN PHOSPHOTRANSFERASE II (HPTII; a 845 bp amplicon) in each assessed S. viridis putative transformant (transformant lines 1 to 5). WT: the non-transformed S. viridis wild-type plant (negative PCR control). P: pANIC12A plasmid DNA (positive PCR control).