Figure 1. Different SLC alternating access transport mechanisms.
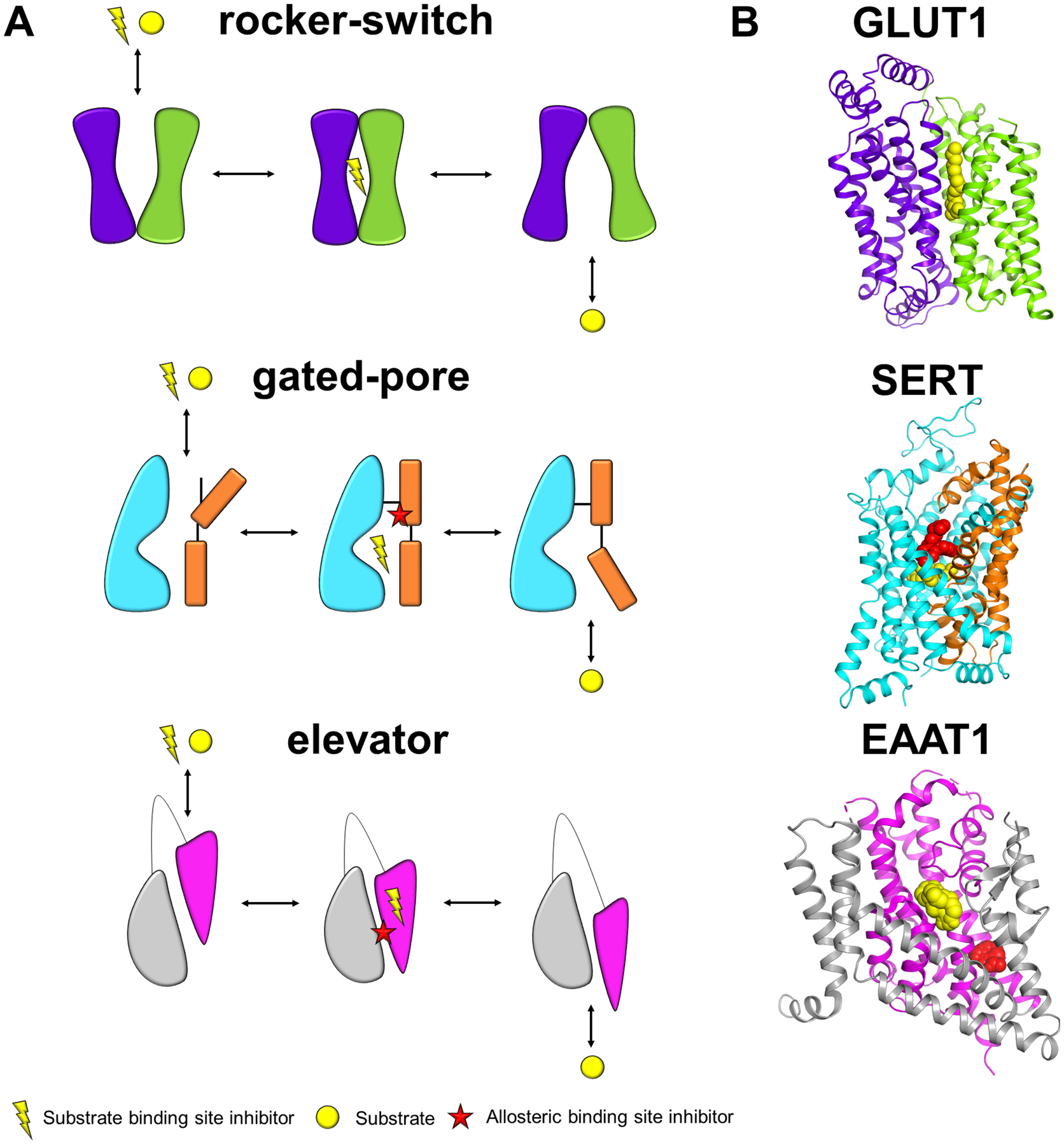
(A) For each transport mechanism, there is a representative schematic of an unbound outward-open conformation that is accessible to both inhibitor and substrate, an occluded inhibitor bound state, representative of when inhibitor binding blocks substrate transport and an inward open conformation, which is accessible to substrate from the intracellular side of the membrane. The colors denote the two different domains involved in the mechanism. The figure only shows one protomer even if more than one subunit is involved. The rocker-switch mechanism, the substrate binds to the extracellular facing binding site, triggering conformational changes to an occluded state, followed by an inward-facing state where the substrate is released. Rocking bundle or gated-pore mechanism has a mobile bundle domain (in orange) that undergoes large hinge-like rearrangements to release the substrate to the intracellular side, while the scaffold domain (in cyan) remains static. The elevator mechanism has a mobile domain (pink) that moves up and down, relative to a scaffold domain (gray), to transport the substrate across the membrane. (B) Representative structures of transporters using the transport mechanisms in (A) where the colors correspond to the respective domains as shown in (A), substrate binding site and allosteric site inhibitors are shown in yellow and red spheres, respectively. PDB IDs: GLUT1: 4PYP, SERT: 5I73, EAAT1: 5LLM.
