Figure 2. Substrate and allosteric binding sites of EAAT1 and ASCT2.
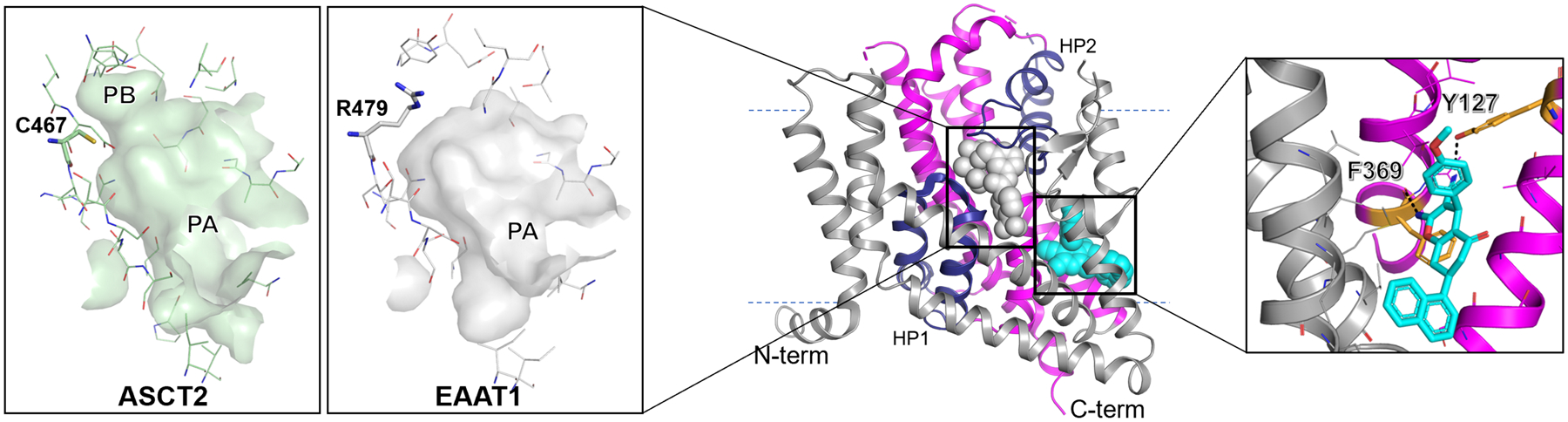
Here, gray represents the scaffold domain and; dark blue hairpins 1 and 2 (HP1 and 2) and pink comprise the transport domain. The substrate and allosteric binding sites of EAAT1 (PDB ID: 5MJU) are shown in gray and cyan spheres respectively. Here, dotted lines show the approximate location of the membrane. Inset left: Surface representations of the substrate binding site of the outward-open conformation for ASCT2 and EAAT1. Pockets A and B (PA and PB) are highlighted and residues impacting substrate specificity and binding site shape are shown as sticks with oxygen, nitrogen, and sulfur atoms shown in red, blue, and yellow. Inset right: The allosteric inhibitor UCPH101 bound to EAAT1. Key residues making polar contacts with UCPH101 are highlighted as orange sticks. Images were generated with PyMOL (https://pymol.org/2/).
