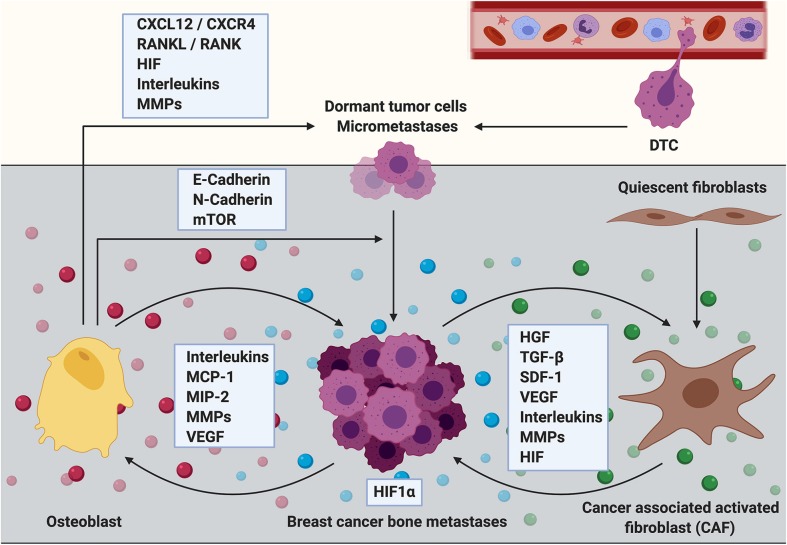
Figure 2.
The role of osteoblasts and cancer associated fibroblasts during the establishment and progression of breast cancer bone metastasis. Cells of mesenchymal origin including osteoblasts and cancer associated fibroblasts (CAFs) are increasingly recognized to contribute to the establishment and progression of breast cancer bone metastasis. Osteoblasts express cytokines including C-X-C motif chemokine ligand 12 (CXCL12, also referred to as stromal derived factor 1, SDF-1) and receptor activator of NF-κB ligand (RANKL) that promote breast cancer cell dissemination and metastatic growth through interaction with their corresponding receptors (CXCR4 and RANK, respectively) that are expressed by the cancer cells. Breast cancer micrometastases have also been shown to be surrounded by osteoblastic cells. The interaction between breast cancer cells and osteoblasts could partially be mediated via heterotypic adherens junctions using E-, and N-cadherins resulting in an enhanced mTOR activity in cancer cells and consequently in the transition from dormant tumor cells into overt metastases. Osteoblasts also express high levels of extracellular matrix remodeling proteins (MMPs) in addition to reduced presence of inflammatory cytokines such as interleukins (ILs) upon cancer cell stimulation. Thereby osteoblasts could regulate breast cancer cell dormancy in the bone microenvironment. In contrast, metastatic breast cancer cells increase the production of inflammatory cytokines such as IL-6 and IL-8, monocyte chemoattractant protein−1 (MCP-1), macrophage—inflammatory protein 2 (MIP-2) and vascular endothelial growth factor (VEGF) in osteoblasts, thereby promoting breast cancer cell invasiveness and metastasis progression. Although usually quiescent in normal tissue, fibroblasts acquire an activated phenotype during processes such as wound healing or inflammation. Activated fibroblasts in the tumor stroma are called cancer associated fibroblasts (CAFs). They produce growth factors that contribute to disease progression including hepatocyte growth factor (HGF), transforming growth factor beta (TGF-β), stromal derived factor 1 (SDF-1 or CXCL12), VEGF, IL-6, and other ILs in addition to MMPs. All of these factors promote primary tumor growth and it can be hypothesized that CAFs could similarly mediate the growth of breast cancer bone metastases. CAFs are known to induce extracellular matrix remodeling and alter the stiffness of tissues thereby facilitating tumor cell invasion, dissemination and/or metastasis. CAF-induced matrix remodeling and CAF invasion have been shown to be supported by hypoxia inducible factor−1 alpha (HIF1α). In turn, an increased expression of HIF1α might stimulate the tumor growth promoting function of CAFs.