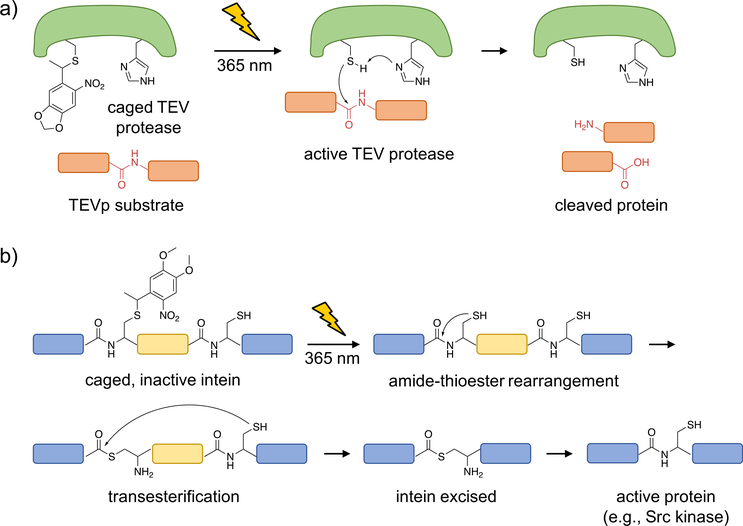
Figure 2. Optical control of TEV protease function and intein splicing using photocaged cysteine.
a) Upon caging of TEVp, the catalytic activity is blocked until photolysis of the caged, catalytically active cysteine, which performs a nucleophilic attack onto the protein substrate to generate cleaved fragments. b) A caged intein, indicated by the region in yellow, can be strategically placed within a protein (shown in blue; e.g., mCherry or Src kinase) such that it is misfolded and inactive prior to irradiation. Upon photo-activation, the native cysteine is generated and the intein is excised, leading to full-length, active protein (with only a cysteine scar).