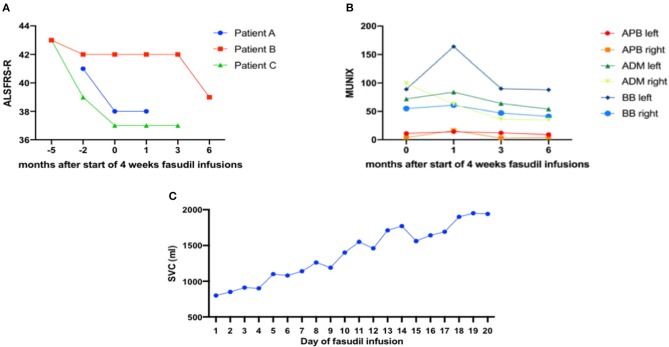
Figure 2.
(A) Revised ALS Functional Rating Scale (ALSFRS-R) was determined at the given time-points before and after 1 month of Fasudil infusions. Patient A and C had a more aggressive disease progression of >1 point decline per month while patient B showed a decline of only 1 point over 3 months. In all three patients, ALSFRS-R progression was attenuated significantly during and shortly after Fasudil infusions. (B) In patient B, Motor unit index (MUNIX) was measured in the given arm muscles before and at the end of 1 month Fasudil infusions as well as 3 and 6 months after the first day of infusion. MUNIX was increased in 5 of 6 measured muscles after 4 weeks of Fasudil treatment. At the follow-up visits at 3 and 6 months, MUNIX gradually decreased again in all measured muscles. APB, abductor pollicis brevis muscle; ADM, adductor digiti minimi muscle; BB, biceps brachii muscle. (C) Patient A had a severely impaired respiratory function with a slow vital capacity (SVC) of only 800 ml (26% of normal) at the start of Fasudil treatment. SVC was measured daily during the 20 consecutive working days of Fasudil infusions. It increased to 1,900 ml at the end of the treatment.