Figure 1.
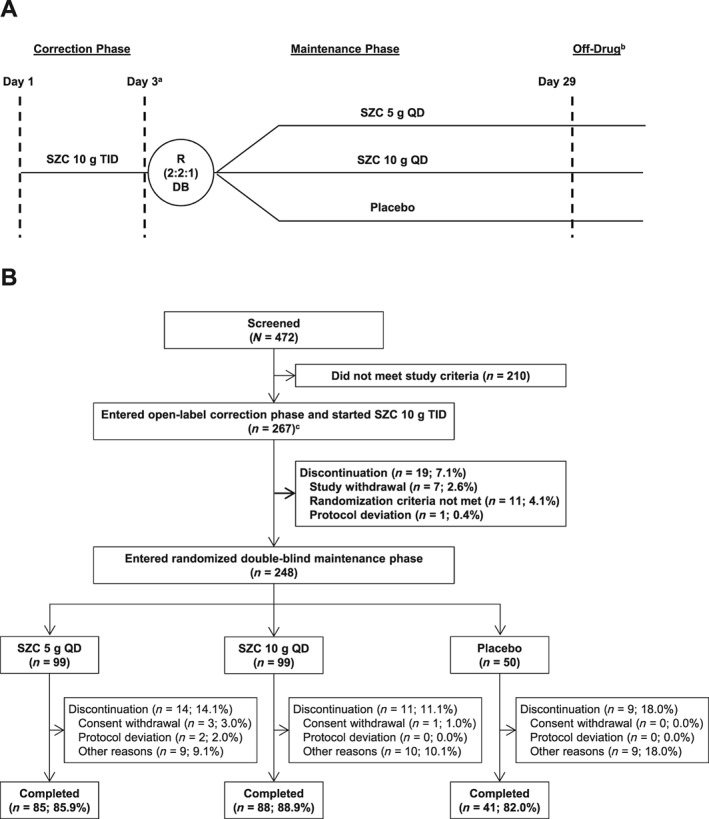
Study design (A) and patient disposition (B). aDay 3 of the correction phase was also considered day 1 of the maintenance phase. bOff‐drug visit occurred within 7 (±1) days after last dose administration. cFive patients who did not meet the study inclusion/exclusion criteria were enrolled in the correction phase. DB, double‐blind; QD, once‐daily; R, randomization; SZC, sodium zirconium cyclosilicate; TID, thrice‐daily.
