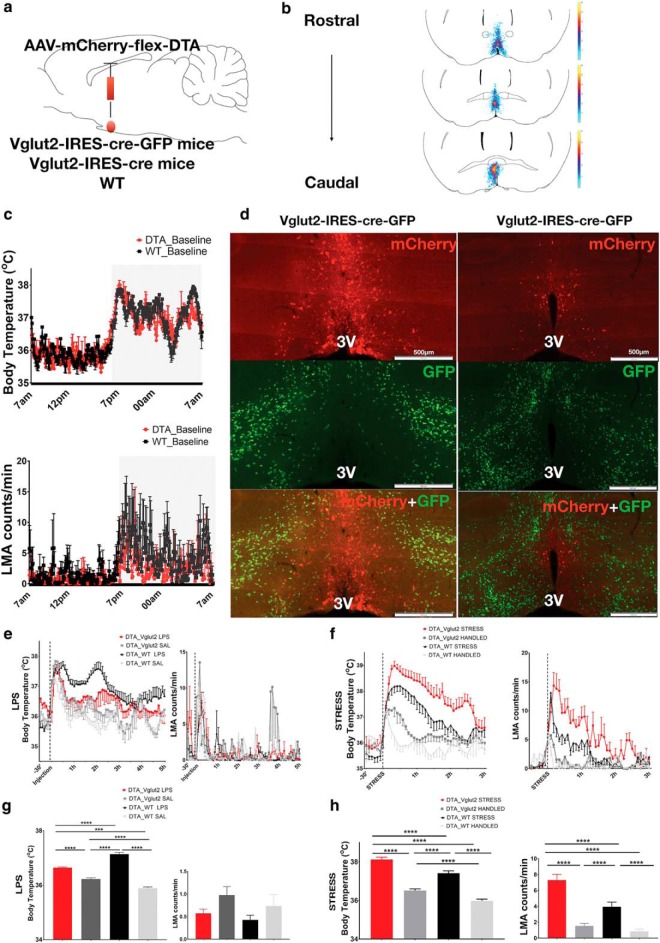
Figure 3.
Ablation of MnPOVglut2 neurons eliminates fever induced by LPS and causes hyperthermia during behavioral stress. a, Schematic figure of AAV-DTA injection in the MnPO of VGluT2-IRES-cre-GFP, VGluT2-IRES-cre, or WT mice. b, A heatmap of the injection sites of all experimental mice. c, Baseline Tb of VGluT2-Cre mice injected with AAV-DTA (n = 6) or WT mice (n = 6) as control (DTA_VGluT2, 36.4 ± 0.03°C; DTA_WT, 36.59 ± 0.04°C; two-way ANOVA, p = 0.78 during 24 h) and baseline locomotor activity of the same mice (DTA_VGluT2, 1.7 ± 0.1 cpm; DTA_WT, 3.0 ± 0.16 cpm; p = 0.80, two-way ANOVA). d, The top panels show the mCherry (red) expression in the non-VGluT2 neurons in the MnPO outlining the AAV injection site, the middle panels show the remained VGluT2 neurons (green) in the POA region of a VGluT2-IRES-cre-GFP reporter mouse, and the bottom panels show the efficiency of AAV-DTA ablation of MnPOVglut2 neurons in two coronal sections from the same VGluT2-IRES-cre-GFP reporter mouse (few if any green VGluT2 neurons are left in the field of red neurons that marks the AAV-DTA injection); no colocalization of VGluT2 neurons (green) and mCherry (red) was seen. e, VGluT2-cre-DTA mice injected with LPS (0.02 mg/kg; n = 6; red) showed minimal fever responses compared with WT-DTA mice injected with same dose of LPS (n = 6; black). However, no difference was found in LMA patterns between groups treated with LPS. f, During the cage exchange stress protocol, VGluT2-cre-DTA mice (red) showed an increase in Tb compared with mice only handled (gray), but not transferred to a dirty cage, and a higher increase in Tb than WT-DTA subjected to same stress protocol (black). In addition, VGluT2-cre-DTA mice handled, but not transferred to a dirty cage, show a greater increase in Tb compared with WT-DTA handled mice (light gray). VGluT2-cre-DTA mice also show a greater increase in locomotion compared with WT-DTA mice during both handling and cage exchange stress. g, During the peak of the fever response, VGluT2-cre-DTA mice show a Tb increase of ∼0.4°C compared with the same mice treated with saline (n = 6), while WT-DTA mice treated with LPS show an increase of ∼1.8°C compared with saline (DTA_VGluT2 LPS, 36.64 ± 0.02°C; DTA_VGluT2 saline, 36.23 ± 0.04°C; DTA_WT LPS, 37.14 ± 0.06°C; DTA_WT saline, 35.91 ± 0.04°C; ***p < 0.001, ****p < 0.0001, F(3,355) = 102.2, two-way ANOVA, followed by Bonferroni's post hoc test; n = 6 DTA_VGluT2 LPS; n = 6 DTA_VGluT2 saline; n = 6 DTA_WT LPS; n = 3 DTA_WT saline), but no changes were found in LMA. h, During the first 2 h of the cage exchange protocol, VGluT2-cre-DTA mice showed an increase in Tb compared with the same mice handled in the same way, but placed in their own cages, and a higher increase in Tb when compared with WT-DTA mice subjected to the same stress protocol. VGluT2-cre-DTA mice handled also show an increase in Tb compared with WT-DTA mice handled in the same way (DTA_VGluT2 STRESS, 38.15 ± 0.12°C; DTA_WT STRESS, 37.47 ± 0.12°C; DTA_VGluT2 Handled, 36.5 ± 0.9°C; DTA_WT HANDLED, 35.98 ± 0.08; ***p < 0.001, ****p < 0.00011, F(4,445) = 280.8, two-way ANOVA, followed by Bonferroni's post hoc test; n = 6 DTA_VGluT2 STRESS; n = 6 DTA_VGluT2 HANDLED; N = 6 DTA_WT STRESS; and N = 3 DTA_WT Handled). VGluT2-cre-DTA mice subjected to the cage exchange protocol also show an increase in locomotion when compared with the same mice when handled or WT-DTA mice during the first 2 h of stress (DTA_VGluT2 STRESS, 7.39 ± 0.7 cpm; DTA_WT STRESS, 3.8 ± 0.5 cpm; DTA_VGluT2 HANDLED, 1.3 ± 0.2 cpm; DTA_WT HANDLED, 0.8 ± 0.2 cpm; ***p < 0.001, ****p < 0.0001, F(3,425) = 89.78 two-way ANOVA, followed by Bonferroni's post hoc test; n = 6 DTA_VGluT2 STRESS; n = 6 DTA_VGluT2_HANDLED; n = 6 DTA_WT STRESS; n = 3 DTA_WT HANDLED). 3V, Third ventricle.