Fig. 3. DG-8 competition assays in A549 cell lysates.
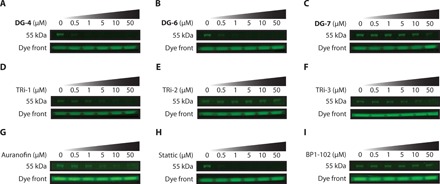
(A to C) To investigate whether top DG compounds shared the same target as DG-8, A549 protein lysates (30 μg) were incubated with DG compounds (0.5 to 50 μM) for 30 min and then with DG-8 (5 μM) for 30 min. Samples were then run on an SDS-PAGE gel, and DG-8 fluorescence was measured using a Gel Doc EZ Gel Documentation System with UV tray. Both DG-4 and DG-5 outcompeted DG-8 for binding at low micromolar concentrations, whereas DG-7 was much less potent. (D to G) Established TrxR1 inhibitors TRi-1, TRi-2, TRi-3, and auranofin were assessed in the DG-8 competition assay. TRi-3 is highly similar to our lead series of compounds and has a 4,5-dichloropyridazinone group, suggesting that it could have the same molecular target(s) as our top compounds. TRi-1 and auranofin inhibit TrxR1 through binding to its Sec residue, whereas TRi-2 is thought to function by a non-Sec binding mechanism. Hence, TRi-1, TRi-3, and auranofin could all compete with DG-8, suggesting that this band may correspond to the 55-kDa selenoprotein TrxR1 by covalently reacting with its Sec residue. (H and I) Two popular STAT3 inhibitors Stattic and BP1-102 were assessed for DG-8 competition. While both Stattic and BP1-102 claim to be direct binders of STAT3 protein in cells, Stattic showed competition with DG-8, indicating that it may share some common STAT3 inhibitory effects as our top DG compounds. BP1-102 was not able to compete with DG-8 for binding to the ~55-kDa band. All SDS-PAGE gels were run under reducing conditions, and the fluorescence of the dye front was used to ensure regular loading.
