We had the opportunity to read the manuscript by Maarten Koper et al [1] and we did it with great interest, as the authors described a rare dual mobility cup (DMC) complication, that is intraprosthetic dislocation (IPD), associated with increased metal ion levels, which has not been described yet. In their discussion, they suggested regular clinical and radiological follow-up in all patients with a dual mobility cup to detect promptly the potential complications of this system. However, in our opinion, a poor analysis of the possible biomechanical and tribological risk factors of IPD and a wrong choice of the femoral stem are the main limitations of Koper's article.
Based on Philippot classification [2], they described a type 1 IPD which occurred 2 years after revision. Late IPD is mainly related to the wear of the retentive rim of the mobile polyethylene liner and the femoral head in the “third joint” [[3], [4], [5]]. Nevertheless, the authors either did not find any macroscopic damage of the polyethylene liner or study the retrieved liner to demonstrate the possible zone of wear that creates the dislocation of the femoral head. Considering tribological studies, it has been demonstrated [6,7] that IPD is due to retaining rim wear and not to a traumatic phenomenon with hip dislocation. IPD is a wear complication from mainly the outer side of the liner-retaining rim. This wear is mainly due to contact between the femoral neck and the outside of the rim. IPD appeared with the first DMC series because a large nonpolished neck was used. When the second-generation DMC was introduced, the rate of IPD has been reported to have an incidence of only 0.1% [8]. This reduction can be explained by the following factors: optimization of the contact between the prosthetic neck and retaining ring, optimization of the chamfer, use of higher molecular weight polyethylene, and change to a polished neck that is trapezoid, elliptical, or circular in shape. This decrease in the incidence of IPD is confirmed by the latest studies of third-generation implants in which no IPD has been reported [9]. The common element of all IPDs is the damage of the capturing area of the polyethylene component related to the impingement of the prosthetic femoral neck against the chamfer. Either homogeneous or circular wear of the retentive mechanism or asymmetric damage secondarily can occur [9]. In the setting of a loose socket, wear may be accelerated. We concluded that the complication described in this case report was a true IPD but we believe that the cause of the IPD was incomplete seating of the prosthetic metal head into the mobile polyethylene component, as previously described by Guyen et al in 2009 [8]. Although there is no industry standard defining the impaction force necessary for seating a femoral head onto a stem or for assembly of dual-mobility articulations, strict adherence to the manufacturer's assembly instructions may reduce the risk of IPD. Before reduction, it is essential to ensure that the head is securely seated on the stem and that the mobile bearing moves freely.
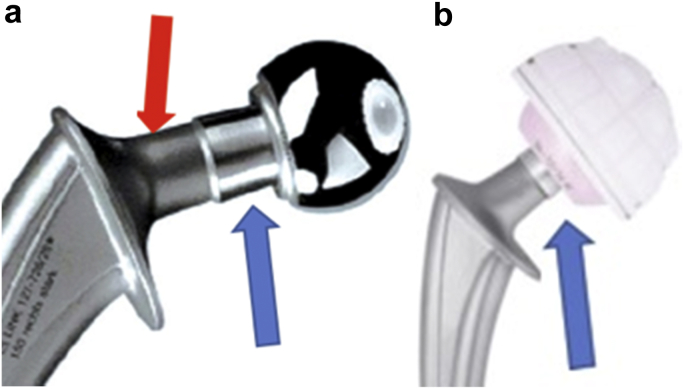
A second concern is about the choice of the cemented Lubinus SP II (Link, Hamburg, Germany) stem at the time of revision. That stem is certainly the most implanted in Northern Europe [10]. Nevertheless, using the Lubinus stem, the contact of the third joint is not optimized due to the long taper and a big and rough neck. The diameter of the Lubinus neck is always greater than 14 mm and its surface roughness is 1.5 μm (10; Fig. 1). In 2001, Noyer conducted a study on the mid-term results on DMC and he was able to demonstrate the role of the design and the surface of the femoral stem neck when using DMC [3]. Revisions for IPD, which occurred on average approximately 4 years after implantation, were twice as likely for rougher necks compared to polished necks. IPD was widely reported with the first-generation designs but had occurred less frequently with “friendly neck” designs. These stems have a highly polished (roughness 0.1 μm) and thinner neck, with a head-neck ratio of at least 2 [11].
Figure 1.
Lubinus stem construct with a 22.2 mm metallic head (a) or a 28 mm ceramic head (b). Blue arrows show that the radius taper is not covered by the head. Red arrow shows the roughness of the neck. (Pictures captured in: From Excellent results with the cemented Lubinus SP II 130-mm femoral stem at 10 years of follow-up; Acta Ortopedica 85; April 2014).
It is instructive to note that the contralateral hip in the reported patient had an isolated revision to a cemented DMC with retention of a polished Charnley stem, which has had favorable reports in combination with dual-mobility articulations [[12], [13], [14], [15]]. We completely disagree with the authors' statement “Our case and review of the literature may form an argument not to consider DMC for primary cases and placement in younger patients should be performed with caution” as the efficacy of contemporary monoblock implants has been reported [[16], [17], [18], [19], [20], [21]]. In addition, results at more than 10 years in THA in patients less than 55 years have been shown to be excellent without an increase in serum cobalt or chromium levels [22]. To prevent IPD, surgeons must not forget the lessons of the past and should carefully choose which implants they choose in dual-mobility constructs.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field whichmay be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2020.01.002.
Appendix A. Supplementary data
References
- 1.Koper M., Verdijk R., Bos K. Asymptomatic intra-prosthetic dual mobility cup dislocation with increased metal ion levels. Case Report. Arthroplasty Today. 2019;5:38. doi: 10.1016/j.artd.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philippot R., Boyer B., Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471:965. doi: 10.1007/s11999-012-2639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noyer D. La troisieme articulation des prothèses de hanche a double mobilite. Maîtrise Orthopédique. 2003;121:20. [Google Scholar]
- 4.Lecuire F., Benareau J., Rubini J. Intra-prosthetic dislocation of the Bousquet dual mobility socket. Rev Chir Orthop Reparatrice Appar Mot. 2004;90:249. doi: 10.1016/s0035-1040(04)70101-4. [DOI] [PubMed] [Google Scholar]
- 5.Tigani D., Prudhon J.L., Amendola L., Aslanian T. Letter to the editor on “Early intraprosthetic dislocation in dual-mobility implants: a systematic review”. Arthroplasty Today. 2017;4(1):132. doi: 10.1016/j.artd.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neri T., Philippot R., Farizon F., Fessy M.H. Polyéthyléne standard – luxation intraprothétique. In: Elsevier Health Sciences, editor. La double mobilité en marche dans les prothèses totales de hanche. Issy-les-Moulineauxcedx; France: 2018. p. 171. [Google Scholar]
- 7.Neri T., Boyer B., Geringer J. Intraprosthetic dislocation of dual mobility total hip arthroplasty: still occurring? Int Orthop. 2019;43:1097. doi: 10.1007/s00264-018-4054-0. [DOI] [PubMed] [Google Scholar]
- 8.Neri T., Philippot R., Klasan A. Dual mobility acetabular cups for total hip arthroplasty: advantages and drawbacks. Expert Rev Med Devices. 2018;15:835. doi: 10.1080/17434440.2018.1538781. [DOI] [PubMed] [Google Scholar]
- 9.Gaillard R., Kenney R., Delalande J.L., Batailler C., Lustig S. Ten- to 16-year results of a modern cementless dual-mobility acetabular implant in primary total hip arthroplasty. J Arthroplasty. 2019;34:2704. doi: 10.1016/j.arth.2019.06.051. [DOI] [PubMed] [Google Scholar]
- 10.Guyen O., Pibarot V., Vaz P., Chevillotte C., Bejui-Hugues J. Use of a dual mobility socket to manage total hip arthroplasty instability. Clin Orthop Relat Res. 2009;467:465. doi: 10.1007/s11999-008-0476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junnila M., Inari Laaksonen I., Eskelinen A. Implant survival of the most common cemented total hip devices from the Nordic Arthroplasty Register Association database. Acta Orthop. 2016;87(6):546. doi: 10.1080/17453674.2016.1222804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdonschot N. In: “Stem design philosophies. Implant choice”. 168-179. ISBN 3-540-24197-3. Breusch S.J., Malchau H., editors. Uitg: Springer Medizin Verlag; Heidelberg, Germany: 2005. [Google Scholar]
- 13.Plummer D.R., Haughom B.D., Della Valle C.J. Dual mobility in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):1. doi: 10.1016/j.ocl.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Mertl P., Combes A., Leiber-WackenheiM F., Fessy M.H., Girard J., Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8:251. doi: 10.1007/s11420-012-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odland N., SierraR J. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):1124. doi: 10.3928/01477447-20141124-90. [DOI] [PubMed] [Google Scholar]
- 16.Caton J.H., Prudhon J.L., Ferreira A., Aslanian T., Verdier R. A comparative and retrospective study of three hundred and twenty primary Charnley type hip replacements with a minimum follow up of ten years to assess wether a dual mobility cup has a decreased dislocation risk. Int Orthop. 2014;38(6):1125. doi: 10.1007/s00264-014-2313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lautridou C., Lebel B., Burdin G., Vielpeau C. Survival of the cementless Bousquet dual mobility cup: minimum 15-year follow-up of 437 total hip arthroplasties. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(8):731. doi: 10.1016/j.rco.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Prudhon J.L., Ferreira A., Verdier R. “Dual mobility cup dislocation rate and survivorship at ten years of follow-up”. Int Orthop. 2013;37(12):2345. doi: 10.1007/s00264-013-2067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Apuzzo M.R., Koch C.N., Esposito C.I., Elpers M.E., Wright T.M., Westrich G.H. “Assessment of damage on a dual mobility acetabular system”. J Arthroplasty. 2016;31(8):1828. doi: 10.1016/j.arth.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caton J.H., Ferreira A. Dual-mobility cup: a new French revolution. Int Orthop. 2017;41:433. doi: 10.1007/s00264-017-3420-7. [DOI] [PubMed] [Google Scholar]
- 21.Darrith B., Courtney P.M., Della Valle C.J. Outcomes of dual mobility components in total hip arthroplasty: a systematic review of the literature. Bone Joint J. 2018;100-B:11. doi: 10.1302/0301-620X.100B1.BJJ-2017-0462.R1. [DOI] [PubMed] [Google Scholar]
- 22.Puch J.M., Derhi G., Descamps L., Verdier R., Caton J.H. Dual mobility in total hip arthroplasty in patients less than 55 years and over 10 years follow up. Int Orthop. 2017;41(3):475. doi: 10.1007/s00264-016-3325-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.