Figure 1.
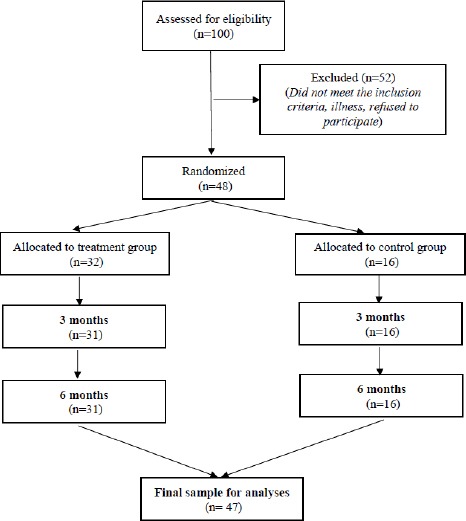
Flow chart of patients from recruitment to the end of 6-month randomized control trial; There were 100 patients assessed for eligibility if they A) were aged from 18 to 60 years and B) had an acute complete SCI; They were excluded, if they A) were under mechanical ventilation; B) had diseases other than SCI (e.g., melanoma within 5 years); C) had an infectious disease (i.e., HIV); body temperature higher than 38°C; D) had a predisposing disease (e.g., thrombocytopenia); E) received cytotoxic drug treatment; F) were participating in a clinical trial within 3 months; G) had current psychiatric illness; H) had a traumatic brain injury related to SCI, or I) who did not provide fully informed consent; Screening based on inclusion and exclusion criteria resulted in 100 participants. Then, the participants were randomly allocated into treatment and control group with the ratio 2: 1, respectively. Of the patients screened, 48 met the inclusion criteria and were allocated groups, with 32 in the treated group and 16 in the control group, which consisted of 18 males and 2 females aged 18-59 years old. One patient in the treatment group withdrew from the study for personal reasons. Finally, 47 patients were included in the analysis, with 31 in the treated group and 16 in the control group
