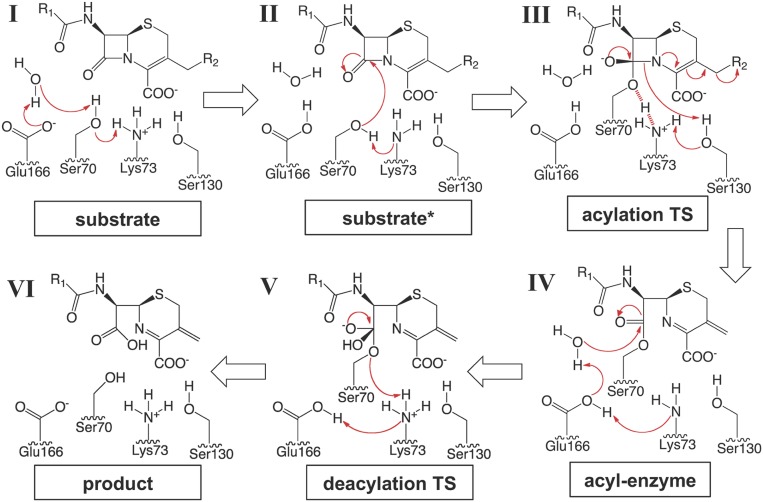
Fig. 6.
A proposed mechanism of proton transfer for the class A β-lactamase catalysis. A cephalosporin is used as a representative β-lactam substrate. Electron transfers are indicated by arrows. Based on QM/MM calculations and experimental data, the reaction of class A β-lactamases follows a concerted base catalysis mechanism involving Lys73 and Glu166. (I) Substrate binding triggers a series of proton transfers, changing the protonation states of both Lys73 and Glu166 from being charged to neutral. (II) A neutral Lys73 deprotonates and activates Ser70 for nucleophilic attack on the β-lactam ring. (III) The reaction proceeds to a high-energy tetrahedral intermediate, where Ser70 is covalently attached to the substrate while being stabilized by a potential LBHB with Lys73 (indicated by red dashed lines). The shared proton of the LBHB is subsequently transferred to Lys73, which protonates Ser130 after the serine residue transfers its own proton to the ring nitrogen atom. Isomerization of the cephalosporin dihydrothiazine ring results in cleavage of the C3 side chain, a reaction unique for many cephalosporins. (IV) A neutral Lys73 takes a proton from Glu166. A negatively charged Glu166 serves as the base for the deacylation process and activates the catalytic water molecule for its attack on the acyl–enzyme linkage. (V) Lys73 protonates Ser70 during the collapse of the tetrahedral deacylation TS and then takes a proton from Glu166. (VI) The protonation states of Lys73 and Glu166 return to those of the free enzyme, and the hydrolyzed product is released.