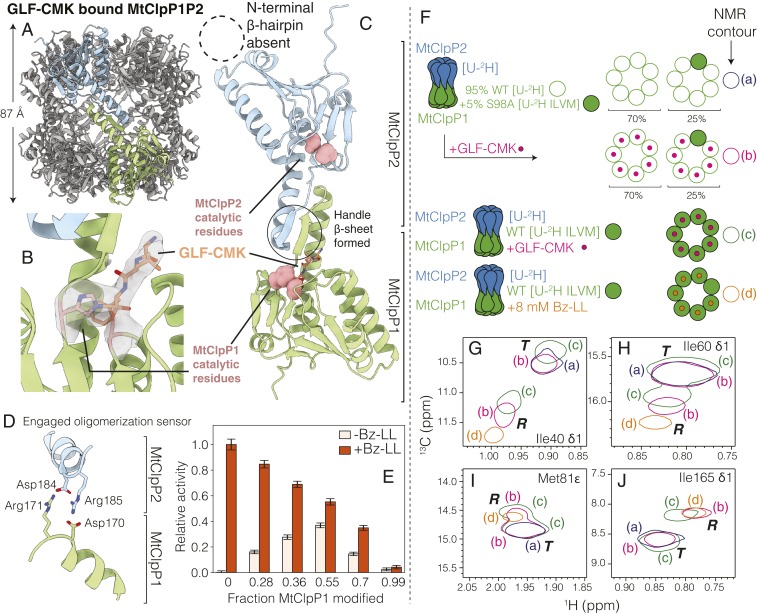
Fig. 5.
Active site inhibitors activate MtClpP1P2 via intraring allosteric communication. (A) Side view of the cryo-EM structure of MtClpP1P2 where the active sites of MtClpP1 are fully modified by GLF-CMK. The height of the MtClpP1P2 complex (excluding the N-terminal β-hairpins of MtClpP2) is noted. (B) Close-up view of the catalytic His and Ser of an MtClpP1 protomer and cryo-EM density for the attached GLF-CMK (both His-123 and Ser-98 of the active site are covalently linked to the inhibitor). (C) Close-up view of a pair of opposite MtClpP1 (green) and MtClpP2 (blue) protomers showing an ordered handle β-sheet (solid circle) as a result of the modification of MtClpP1 by GLF-CMK. Notably, the N-terminal region of MtClpP2 (dotted circle) remains disordered. (D) Status of the oligomerization sensor of MtClpP1P2 in the GLF-CMK–modified form. Side chains of residues involved in salt bridge formation are indicated. (E) Activity of MtClpP1P2 complexes unmodified and partially modified with GLF-CMK assayed with 250 µM PKM-AMC substrate in the absence (white) and presence (orange; 8 mM) of Bz-LL (40 °C). The extent of the modification of the MtClpP1 ring of the MtClpP1P2 complex by GLF-CMK measured from intact protein mass spectrometry (SI Appendix, Fig. S9) is noted on the horizontal axis. Error bars correspond to 1 SD from the average of three replicates. (F) Cartoons of MtClpP1P2 complexes with spectra that are subsequently shown in G–J, indicating the labeling schemes used, and bound ligands. Complexes were generated where either all MtClpP1 protomers were WT or hybrid, with rings produced by mixing 95% [U-2H] WT (open green circles; each circle corresponds to an MtClpP1 protomer) and 5% ILVM-labeled S98A protomers (filled green circles). In the latter case, MtClpP1 heptamers with distributions of WT and S98A subunits were produced, with the dominant configurations shown. These complexes were either subsequently reacted with the GLF-CMK active site inhibitor (pink circles) or not reacted; note that only WT protomers can react. Complexes were also obtained with all WT MtClpP1 protomers either GLF-CMK reacted (pink) or partially Bz-LL bound (8 mM; orange circles; with approximately six Bz-LL molecules bound to MtClpP1 at 8 mM as indicated). The color schematic for G–J is displayed. (G–J) Isolated regions of 1H-13C HMQC spectra (40 °C, 18.8 T) of selected residues from ILVM-labeled MtClpP1 in MtClpP1P2 complexes (WT 2H-labeled MtClpP2). Subspectra of different complexes are shown as discussed in F. Correlations from R and T states are indicated along with resonance assignments for each panel. Spectra are displayed as single contours to avoid cluttering.