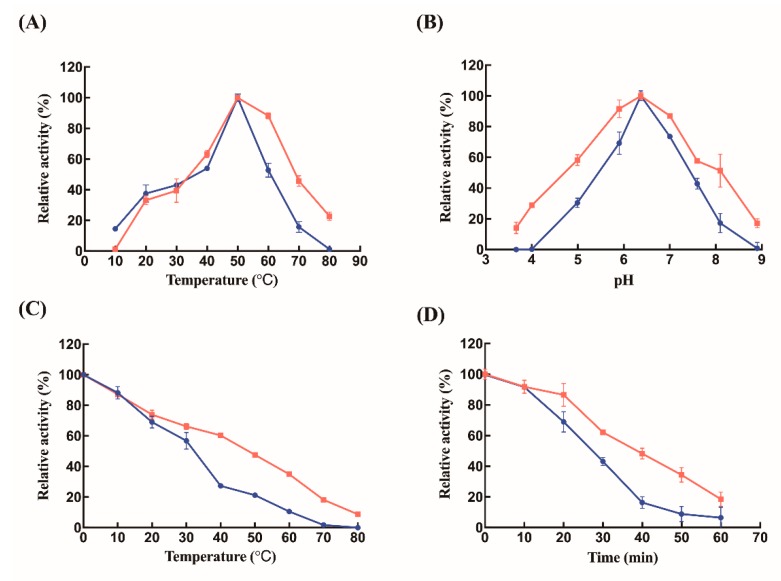
Figure 3.
Biochemical characterization of free (blue circles) and immobilized (red squares) lysozyme. (A) Optimal temperature of the free lysozyme and immobilized lysozyme. The activities were determined in 20 mM sodium phosphate buffer (pH 6.5) for different temperatures from 10 to 80 °C. (B) The effect of pH on the activities of free lysozyme and immobilized lysozyme. Enzymatic activity assay was processed at 50 °C in various buffers as follows: 20 mM sodium acetate buffer (pH 3.7–5.0), 20 mM phosphate buffer (pH 6.0–7.0), and 20 mM glycine-NaOH buffer (pH 7.6–8.9). (C) Thermal stability of free lysozyme and immobilized lysozyme. Enzyme was incubated at various temperatures without substrate for 30 min and then its residual activities assayed at 50 °C and pH 6.5. (D) Thermo-stability of free and immobilized lysozyme at 37 °C for incubation at different times. The enzymatic activity of a fresh sample of free or immobilized lysozyme measured in 20 mM sodium phosphate buffer and at 50 °C was defined as 100%.