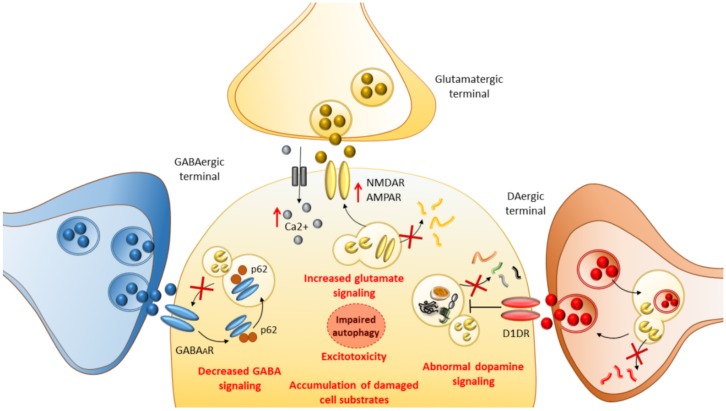
Figure 1.
The role of autophagy in seizure-related molecular mechanisms. By operating at the level of gamma-aminobutyric acid (GABA), dopamine (DA) and glutamate systems autophagy are critically implicated in the molecular mechanisms underlying epileptogenesis and seizure-induced neuronal alterations. When a failure of autophagy occurs, elevated p62 hinders GABAA receptor surface presentation, leading to decreased GABA signaling. At the same time, a failure of autophagy occludes the degradation of glutamate receptors N-Methyl-D-Aspartate (NMDAR) and A-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid (AMPAR), fostering abnormal glutamate signaling and Ca2+ influx. This eventually leads to imbalanced excitatory–inhibitory neurotransmission underlying epileptogenesis. At the level of DA terminals, autophagy is seminal to blunt DA release by degrading DA-filled synaptic vesicles. Autophagy failure leads to abnormal DA release and abnormal stimulation of DA D1 receptors (D1DR), which in turn exacerbate autophagy suppression via mTOR hyperactivation. This eventually leads to the accumulation of damaged cell substrates which synergize with glutamate-related excitotoxicity to produce neuronal damage.