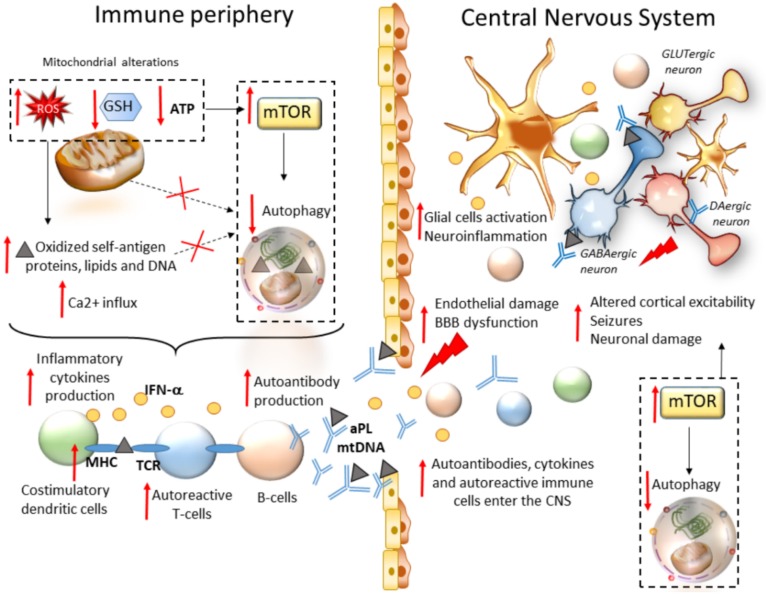
Figure 2.
mTOR-dependent seizure development in autoimmune systemic disorders. Within peripheral immune cells, mitochondrial alterations lead to chronic oxidative stress (production of reactive oxygen species (ROS)) along with a concomitant depletion of antioxidant factors (gluthatione, GSH). This promotes oxidation of self-antigen proteins, lipids, and DNA, along with calcium (Ca2+) influx and the release of highly diffusible oxidative and inflammatory factors, which are spread to other intracellular organelles and through the bloodstream. At the same time, redox imbalance induces mTOR activation and autophagy impairment, eventually leading to impaired removal of oxidized self-antigens and mitochondria, production of inflammatory cytokines by costimulatory dendritic cells, increased stability of major histocompatibility molecules (MHC) on dendritic cells, subsequent activation of autoreactive T cells and production of autoantibodies by B cells. Autoantibodies, activated immune cells, and proinflammatory cytokines are spread through the bloodstream, and they reach the CNS where they produce endothelial damage and disruption of the blood–brain barrier (BBB). Within the brain milieu, these factors, coupled with mTOR hyperactivity and autophagy, impairment promote a chain of events consisting of neuroinflammation through activation of glial cells, altered cortical excitability, and eventually neuronal damage.