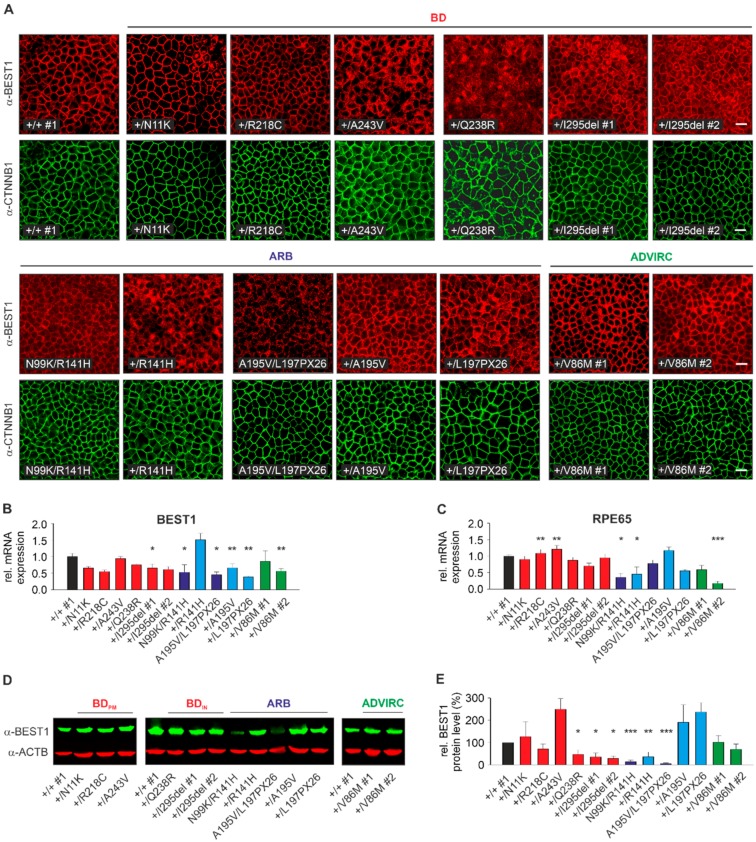
Figure 2.
Localization, RNA, and protein expression analysis of normal and mutated BEST1. (A) Confocal immunofluorescence images of monolayers from control, BD, ARB, and ADVIRC hiPSC-RPEs after five weeks growth on Corning Transwell filter inserts using α-BEST1 and α-β-catenin antibodies. Scale bar: 20 µm. (B–C) Quantification of BEST1 (B) and RPE65 (C) expression by qRT-PCR of total mRNA extracted from BD, ARB, and ADVIRC hiPSC-RPEs relative to control (+/+ #1). Independent samples (n = 3) were measured in triplicates and normalized to HPRT1. Data were given as mean ± SD. Please note that Supplementary Figure S2 depicts individual variation of quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) measurements between individual control cell lines. (D) Representative Western blot images of whole cell lysates from control, BD, ARB, and ADVIRC hiPSC-RPEs using α-BEST1 antibody. Anti-β-actin was used as a loading control. (E) Quantification of BEST1 protein expression from (D) relative to control (+/+ #1) and normalized against β-actin from the same blot. Mean values are given as mean ± SD from two technical replicates per individual sample (n = 2–3). A summary of hiPSC clones used in the experiments is given in Supplementary Table S4. Statistical analyses were performed applying Kruskal-Wallis test, following post-hoc Dunn’s multiple comparisons test including Benjamini-Hochberg procedure: * = p < 0.05; ** = p < 0.01; *** = p < 0.001 and indicated relative to control (+/+ #1).