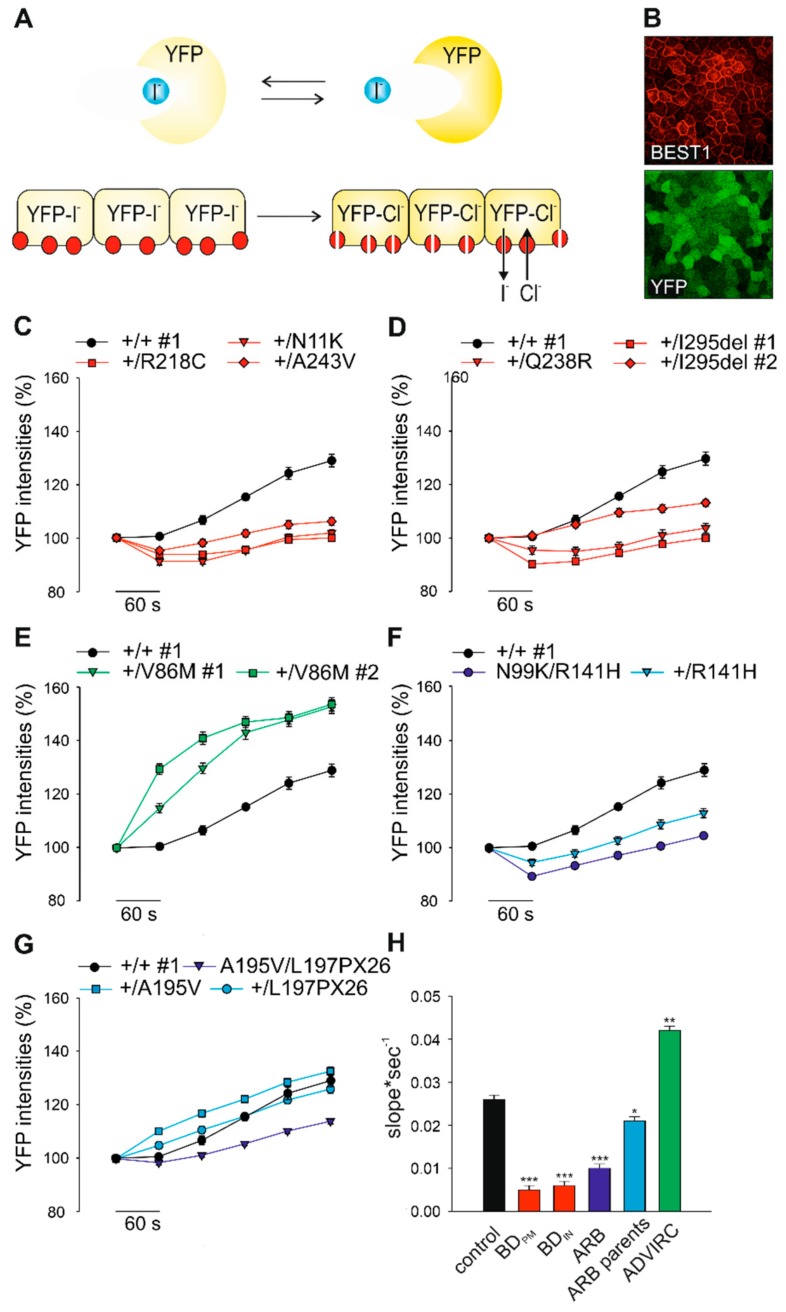
Figure 3.
Consequences of genotype-specific BEST1 mutations on anion permeability in hiPSC-RPEs. (A) Schematic illustration of the yellow fluorescence protein (YFP)-halide transport assay. Human iPSC-RPEs were transduced with YFP-expressing lentiviral particles. YFP fluorescence signals are quenched after addition of iodide ions (I-) and fully reversed after exchanging I- with equimolar chloride ion (Cl-) solution. (B) Fluorescence micrograph of hiPSC-RPEs expressing YFP and normal BEST1 after immunostaining with an α-BEST1 antibody. Scale bar: 20 µm. (C–G) Kinetic of YFP fluorescence intensities over time in hiPSC-RPEs from representative control +/+ #1 and patients associated with BDPM (C), BDIN (D), ADVIRC (E), and the two ARB families (F and G). Recordings were taken at indicated time points after initial exposure to I- followed by the addition of Cl- containing solution. (H) Averaged bar graphs obtained from (C–G) showing recovery rates (slope*sec-1) from initial levels of YFP quenching to maximum fluorescence signals after 5 min of exposure to Cl-. Mean values are given as mean ± SE from six to 18 technical replicates per individual sample (n = 3–4). A summary of hiPSC clones used in the experiments is given in Supplementary Table S4. Statistical analysis was performed applying Kruskal-Wallis test, following post-hoc Dunn’s multiple comparisons test including Benjamini-Hochberg procedure: * = p < 0.05; ** = p < 0.01; *** = p < 0.001 and indicated relative to control.