Abstract
OBJECTIVE
To evaluate the contemporary prevalence of diabetic peripheral neuropathy (DPN) in participants with type 1 diabetes in the T1D Exchange Clinic Registry throughout the U.S.
RESEARCH DESIGN AND METHODS
DPN was assessed with the Michigan Neuropathy Screening Instrument Questionnaire (MNSIQ) in adults with ≥5 years of type 1 diabetes duration. A score of ≥4 defined DPN. Associations of demographic, clinical, and laboratory factors with DPN were assessed.
RESULTS
Among 5,936 T1D Exchange participants (mean ± SD age 39 ± 18 years, median type 1 diabetes duration 18 years [interquartile range 11, 31], 55% female, 88% non-Hispanic white, mean glycated hemoglobin [HbA1c] 8.1 ± 1.6% [65.3 ± 17.5 mmol/mol]), DPN prevalence was 11%. Compared with those without DPN, DPN participants were older, had higher HbA1c, had longer duration of diabetes, were more likely to be female, and were less likely to have a college education and private insurance (all P < 0.001). DPN participants also were more likely to have cardiovascular disease (CVD) (P < 0.001), worse CVD risk factors of smoking (P = 0.008), hypertriglyceridemia (P = 0.002), higher BMI (P = 0.009), retinopathy (P = 0.004), reduced estimated glomerular filtration rate (P = 0.02), and Charcot neuroarthropathy (P = 0.002). There were no differences in insulin pump or continuous glucose monitor use, although DPN participants were more likely to have had severe hypoglycemia (P = 0.04) and/or diabetic ketoacidosis (P < 0.001) in the past 3 months.
CONCLUSIONS
The prevalence of DPN in this national cohort with type 1 diabetes is lower than in prior published reports but is reflective of current clinical care practices. These data also highlight that nonglycemic risk factors, such as CVD risk factors, severe hypoglycemia, diabetic ketoacidosis, and lower socioeconomic status, may also play a role in DPN development.
Introduction
Diabetic neuropathy is a prevalent complication in patients with diabetes and a major cause of morbidity and mortality (1). Among the various forms of diabetic neuropathy, distal symmetric polyneuropathy (DPN) and diabetic autonomic neuropathies are by far the most studied (1).
DPN is one of the most important causes of foot ulceration (2) and lower extremity amputations in the U.S. Patients with DPN and prior lower-extremity amputations have a 50% higher risk for losing a second limb within the next 2 years and 5-year survival rates that are substantially lower than age- and sex-matched patients with diabetes without DPN (1–3). DPN has multiple other consequences, such as major impact on impaired daily function through small- and large-nerve fiber dysfunction, as well as loss of sensory perception, including proprioception, temperature discrimination, and pain, all of which ultimately lead to unsteadiness; recurrent minor injuries, with an increased risk of falls and fractures (1,4); impact on daily function (1,4); impaired control of the accelerator pedal while driving (5); poor oral health (6); and poor quality of life (1,7). Thus, the clinical and socioeconomic costs of DPN are staggering.
Prior estimates of the incidence and prevalence of DPN in the adult population with type 1 diabetes have been obtained through interventional and observational studies, including the follow-up of the Diabetes Control and Complications Trial/Epidemiology and Diabetes Interventions and Complications (DCCT/EDIC) or the Epidemiology of Diabetes Complications Study (EDC) cohorts, and these estimates vary greatly (1,8,9). Yet, there are continuous changes in the standard of care and differences in the access to medical care across the U.S. (10). Thus the objectives of this study were to assess the contemporary prevalence of and potential risk factors and comorbidities for DPN in U.S. adults with type 1 diabetes.
Research Design and Methods
Participants and Data Source
The T1D Exchange Clinic Network includes >80 U.S.-based pediatric and adult endocrinology practices. Details on eligibility criteria, the informed consent process, and data collection have been published previously (11). More than 25,000 individuals with type 1 diabetes were enrolled between September 2010 and August 2012. Core data were updated annually from medical record data extraction. This study targeted U.S. adults ≥18 years with ≥5 years type 1 diabetes duration, participating and receiving care from a clinic participating in the T1D Exchange Clinic Network. This report includes data on all adult participants from those sites in the T1D Exchange Clinic Network who met inclusion criteria and who completed the Michigan Neuropathy Screening Instrument questionnaire (MNSIQ) between April 2016 and April 2018.
Demographic data on sex, race/ethnicity, insurance, annual household income, education level, smoking status, the occurrence of severe hypoglycemia (SH), defined as seizure and/or loss of consciousness, in the prior 3 months, and the occurrence of diabetic ketoacidosis (DKA) in the prior 3 months were collected through comprehensive participant questionnaires. Information about age, diabetes duration, diabetes control as estimated from the HbA1c levels, insulin treatment (insulin pump or multiple daily injections), use of a continuous glucose monitor (CGM), blood pressure, height, weight, smoking status, chronic complications, serum creatinine level, retinopathy, cardiovascular disease (CVD), lipid levels, and use of medications were obtained as part of usual care and were collected from clinic medical records.
Assessment of DPN
Evaluation of the presence of DPN was performed with the MNSIQ, which includes 15 self-administered “yes” or “no” questions on foot sensation, including pain, numbness, and temperature sensitivity, that is scored by summing responses that show an abnormality (12). A score of ≥4, which has been validated to be specific and sensitive for the presence of DPN (13), was used to define the presence of DPN. A secondary outcome of painful DPN was defined as selection of “yes” to the MNSIQ question: “Do you ever have any burning pain in your legs and/or feet?” Calculation of estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration equation (14).
Statistical Analysis
Stepwise logistic regression models were used to assess the association between DPN and age, sex, race/ethnicity, diabetes duration, HbA1c, education level, insurance type, smoking status, geographical region, BMI, height, diastolic and systolic blood pressure, total daily insulin, CGM use, insulin pump use, LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), and triglycerides. Logistic regression models were used to assess the association between DPN and the following medications, medical conditions, measurements and events, adjusting for covariates that remain in the above stepwise regression model: statin medication use; ACE inhibitor/angiotensin II receptor blocker (ARB) medication use; presence of retinopathy, foot ulcers, Charcot neuroarthropathy, carpal tunnel, and CVD (defined as history of coronary artery disease, myocardial infarction, atherosclerotic peripheral vascular disease and heart failure); eGFR; occurrence of one or more SH events; and occurrence of one or more DKA events.
Stepwise logistic regression models were used to assess the association between painful DPN and sex, HbA1c, BMI, LDL-C, HDL-C, and triglycerides. The association between painful DPN and the following medical conditions, measurements, and events were assessed through logistic regression models adjusted for covariates that remain in the prior stepwise regression model: presence of foot ulcers, Charcot arthropathy, carpal tunnel, CVD, eGFR, occurrence of one or more SH events, and occurrence of one or more DKA events.
If a lipid or blood pressure covariate was selected from stepwise regression, then statin medication use also was included as a covariate in the respective logistic regression model. Multiple comparisons were corrected using the adaptive Benjamini-Hochberg false discovery rate correction method (15). Results are expressed as mean ± SD for normally distributed variables or median and interquartile range (IQR) for nonnormally distributed variables. Data analyses were performed using SAS 9.4 software. All P values are two-sided.
Results
Baseline Characteristics
Data were obtained from 5,936 eligible T1D Exchange participants from 63 sites across the U.S. As reported in Table 1, mean age of responders was 39 ± 18 years, 55% were women, and 88% were non-Hispanic white. The median duration of type 1 diabetes was 18 (IQR 11, 31) years, and the mean HbA1c was 8.1 ± 1.6% (65.3 ± 17.7 mmol/mol). An insulin pump was used by 66% of participants and a CGM by 31%. In this cohort, 67% had at least an associate degree, 56% had an annual income of ≥$75,000, and 80% had access to private insurance (Table 1).
Table 1.
Characteristics of T1D Exchange Registry cohort
| Characteristic | Overall (N = 5,936) | No DPN (n = 5,306) | MNSIQ-DPN (n = 630) | P value† |
|---|---|---|---|---|
| Age (years) | 39 ± 18 | 37 ± 17 | 51 ± 17 | <0.001 |
| Female* | 55 | 54 | 60 | <0.001 |
| Race/ethnicity* | 0.05 | |||
| Non-Hispanic white | 88 | 88 | 89 | |
| Non-Hispanic black | 3 | 3 | 5 | |
| Hispanic/Latino | 5 | 6 | 3 | |
| Other race/ethnicity | 3 | 3 | 3 | |
| Highest education level* | <0.001 | |||
| Less than high school graduate | 3 | 2 | 5 | |
| High school graduate/GED | 31 | 29 | 40 | |
| Associate or bachelor degree | 42 | 43 | 37 | |
| Master, professional, or doctorate degree | 25 | 25 | 17 | |
| Annual household income* | ||||
| <$50,000 | 29 | 27 | 47 | |
| $50,000 to <$75,000 | 15 | 15 | 15 | |
| ≥$75,000 | 56 | 59 | 38 | |
| Insurance* | <0.001 | |||
| Private insurance | 80 | 83 | 58 | |
| Other insurance | 19 | 17 | 42 | |
| No insurance | <1 | <1 | <1 | |
| Type 1 diabetes duration (years)* | 18 (11, 31) | 17 (11, 29) | 32 (17, 43) | <0.001 |
| HbA1c (%)* | 8.1 ± 1.6 | 8.1 ± 1.6 | 8.4 ± 1.7 | <0.001 |
| HbA1c (mmol/mol)* | 65.3 ± 17.7 | 64.9 ± 17.6 | 68.4 ± 18.6 | <0.001 |
| Total daily insulin use (units/kg)* | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.93 |
| BMI (kg/m2)* | 27.2 ± 5.3 | 27.0 ± 5.2 | 28.5 ± 6.1 | 0.009 |
| Systolic blood pressure (mmHg)* | 123 ± 14 | 123 ± 14 | 127 ± 17 | 0.40 |
| Diastolic blood pressure (mmHg)* | 73 ± 10 | 73 ± 9 | 71 ± 10 | 0.54 |
| eGFR (mL ∙ min−1/1.73 m2)* | 94.8 ± 28.5 | 97.0 ± 27.6 | 79.3 ± 30.3 | 0.02 |
| Triglycerides (mg/dL)* | 98 ± 68 | 96 ± 65 | 115 ± 86 | 0.002 |
| HDL-C (mg/dL)* | 62 ± 19 | 63 ± 19 | 61 ± 18 | 0.49 |
| LDL-C (mg/dL)*† | 92 ± 29 | 93 ± 29 | 89 ± 33 | 0.38 |
| Total cholesterol (mg/dL)* | 173 ± 36 | 173 ± 36 | 173 ± 39 | |
| Medication use | ||||
| ACE inhibitor/ARB* | 26 | 24 | 45 | 0.74 |
| Statin | 35 | 32 | 62 | 0.009 |
| Smokers* | 4 | 4 | 8 | 0.008 |
| Retinopathy | 24 | 21 | 47 | 0.004 |
| Charcot neuroarthropathy | <1 | <1 | 4 | 0.002 |
| CVD | 7 | 5 | 24 | 0.002 |
| Insulin pump use* | 66 | 67 | 62 | 0.70 |
| CGM use* | 31 | 32 | 27 | 0.10 |
| Subjects with ≥1 SH events‡ | 7 | 7 | 14 | 0.04 |
| Subjects with ≥1 DKA events*§ | 3 | 3 | 7 | <0.001 |
All data are presented as the mean ± SD, median (25th, 75th quartiles), or percentage of participants.
Sex missing for 8 participants, race/ethnicity missing for 15 participants, education level missing for 314 participants, annual household income missing for 1,435 participants, insurance missing for 159 participants, type 1 diabetes duration missing for 1 participant, HbA1c missing for 988 participants, total daily insulin use missing for 2,682 participants, BMI missing for 720 participants, systolic and diastolic blood pressure missing for 136 participants, eGFR missing for 2,326 participants, triglycerides missing for 2,465 participants, HDL-C missing for 2,436 participants, LDL-C missing for 2,180 participants, total cholesterol missing for 3,118 participants, ACE inhibitor/ARB use missing for 15 participants, smoking status missing for 216 participants, pump use status missing for 82 participants, CGM use status missing for 156 participants, and DKA events missing for 5 participants.
From a logistic regression model adjusting for age, sex, HbA1c, diabetes duration, level of education, insurance, smoking status, BMI, height, triglycerides, and statin use. P values were adjusted for multiple comparisons via the adaptive false discovery rate correction procedure.
Defined as self-report of a severe hypoglycemia event in the past 3 months.
Defined as self-report of a DKA event in the past 3 months.
Prevalence and Characteristics of Participants With and Without DPN
The overall prevalence of MNSIQ-defined DPN based on a score of ≥4 was 11%. Compared with participants without DPN, participants with DPN were older (51 ± 17 vs. 37 ± 17 years, P < 0.001), had longer type 1 diabetes duration (median 32 vs. 17 years, P < 0.001), and had a higher mean HbA1c (8.4 ± 1.7% vs. 8.1 ± 1.6% [68.4 ± 18.6 vs. 64.9 ± 17.6 mmol/mol], P < 0.001), as reported in Table 1. Within different age strata, DPN prevalence increased with age, ranging from 4% in those 18–25 years old, to 8% in those 26–49 years old, and to 21% in participants >50 years. DPN participants were also more likely to be women (60% vs. 54%, P < 0.001), have lower eGFR (79.3 ± 30.3 vs. 97.0 ± 27.6 mL ∙ min−1/1.73 m2, P = 0.02), and have higher BMI (28.5 ± 6.1 vs. 27.0 ± 5.2, P = 0.009) than non-DPN participants (Table 1). Women had a DPN prevalence of 12% compared with 9% in men. There were no differences in insulin pump or CGM use between the two groups (Table 1). Occurrence of an SH event (14% in participants with DPN vs. 7% in those without, P = 0.04) and a DKA event (7% in participants with DPN vs. 3% in those without, P < 0.001) in the past 3 months were both more common in DPN participants compared with non-DPN participants (Table 1). Prevalence of gastroparesis was higher in participants with DPN than in participants with no DPN regardless of whether or not they had a DKA or SH event (data not shown).
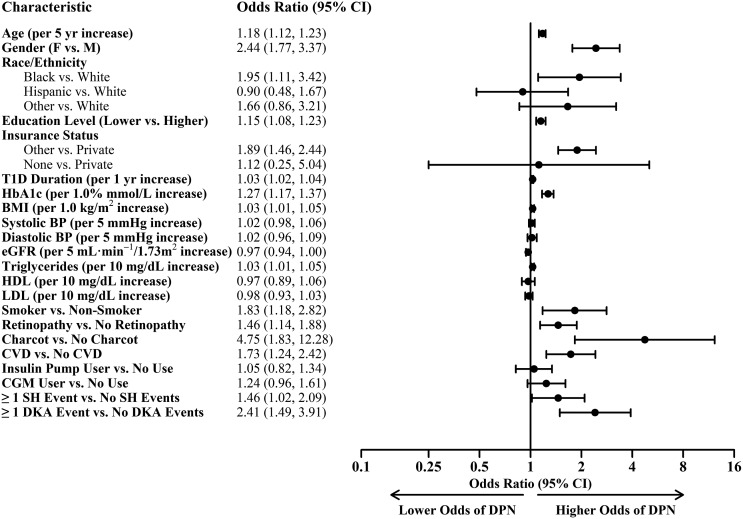
DPN participants had a high prevalence of comorbid CVD compared with non-DPN participants (24% vs. 5%, P = 0.002). Several cardiovascular risk factors were associated with DPN, including higher triglycerides (115 ± 86 vs. 96 ± 65 mg/dL, P = 0.002) and higher rates of smoking (8% vs. 4%, P = 0.008), as reported in Table 1. Odds ratios (OR) of the association of various factors with DPN are shown in Fig. 1.
Figure 1.
ORs ratios of MNSIQ-defined DPN risk factors were calculated from a logistic regression model adjusting for age, sex, level of education, insurance, diabetes duration, HbA1c, BMI, height, triglycerides, statin use, and smoking status. BP, blood pressure; F, female; M, male; T1D, type 1 diabetes.
Novel findings in these analyses highlight the impact of socioeconomic factors on the risk of DPN, including race/ethnicity (black vs. white, OR 1.95 [95% CI 1.11–3.42]), lower education (OR 1.15, [95% CI 1.08–1.23]), and lack of private insurance (other insurance vs. private insurance; OR 1.89 [95% CI 1.46–2.44]) (Fig. 1). Income was evaluated but not included as a possible covariate in the stepwise regression model due to the presence of missing responses and moderate correlation with education.
Painful DPN and Neuropathy Pain Medication Use
Lastly, we evaluated the phenotypes of painful DPN in this cohort. Of the 630 participants with DPN, 427 (68%) reported burning foot pain and were designated as painful DPN. Compared with painless DPN, participants with painful DPN had higher HbA1c (8.5 ± 1.8% vs 8.2 ± 1.6% [69.3 ± 19.2 vs. 66.4 ± 17.1 mmol/mol], P = 0.04) (Table 2) but no other differences between groups were found.
Table 2.
Characteristics of participants with and without painful DPN
| Painful DPN (n = 427) | Painless DPN (n = 203) | P value* | |
|---|---|---|---|
| Age (years) | 52 ± 16 | 51 ± 19 | |
| Female sex | 62 | 57 | 0.21 |
| HbA1c (%)† | 8.5 ± 1.8 | 8.2 ± 1.6 | 0.04 |
| HbA1c (mmol/mol)† | 69.3 ± 19.2 | 66.4 ± 17.1 | 0.04 |
| BMI (kg/m2)† | 28.6 ± 6.2 | 28.2 ± 5.9 | 0.55 |
| Triglycerides (mg/dL)† | 122 ± 95 | 100 ± 57 | 0.11 |
| HDL-C (mg/dL)† | 61 ± 18 | 62 ± 18 | 0.64 |
| LDL-C (mg/dL)† | 91 ± 35 | 83 ± 28 | 0.47 |
| Total cholesterol (mg/dL)† | 177 ± 40 | 163 ± 35 | |
| eGFR (mL ∙ min−1/1.73 m2)† | 81.1 ± 29.2 | 75.2 ± 32.3 | 0.24 |
| Foot ulcers | <1 | 2 | 0.56 |
| Charcot neuroarthropathy | 3 | 6 | 0.47 |
| CVD | 22 | 28 | 0.08 |
| Subjects with ≥1 SH events‡ | 14 | 14 | 0.77 |
| Subjects with ≥1 DKA events†§ | 8 | 4 | 0.12 |
All data are presented as mean ± SD or percentage of participants.
From a logistic regression model adjusting for HbA1c, triglycerides, and statin medications. P values were corrected for multiple comparisons via the adaptive false discovery rate correction procedure.
HbA1c missing for 115 participants, BMI and weight missing for 98 participants, triglycerides missing for 242 participants, HDL-C missing for 245 participants, LDL-C missing for 211 participants, total cholesterol missing for 355 participants, eGFR missing for 173 participants, and DKA missing for 1 participant.
Defined as self-eport of a severe hypoglycemia event in the past 3 months.
Defined as self-report of a DKA event in the past 3 months.
Of the 630 MNSIQ-defined DPN participants, 182 (29%) were taking medications for neuropathy (data not shown). Evaluated medications included amitriptyline, gabapentin, duloxetine, and nortriptyline.
Conclusions
In this study we assessed the prevalence of DPN and painful DPN in a large cohort of patients with type 1 diabetes in the U.S. By using a validated survey questionnaire, we found a DPN prevalence of 11% after a median of 32 years of diabetes duration. Risk factors for DPN in this cohort include traditional DPN risk factors such as older age, longer duration of diabetes, poor glycemic control, female sex, other microvascular complications, established CVD, and CVD risk factors, including BMI, smoking history, and high triglycerides. However, the analyses also unveiled a strong association of DPN with acute diabetes complications, including SH events and DKA events, as well as a correlation between DPN and several socioeconomic factors. Neither of these associations was described before. To our knowledge, this study, which includes >5,900 participants, is the largest study of DPN prevalence in a contemporary population of adults with type 1 diabetes.
Prior interventional and observational studies evaluating DPN in type 1 diabetes, including the DCCT/EDIC, EDC, and EURODIAB, found prevalence rates up to 34% after an average of 25 years of diabetes duration (1,8,9,16,17). The DCCT/EDIC played a pivotal role in our understanding of metabolic memory, which highlighted the importance of early tight glycemic control in patients with type 1 diabetes (9). However, no recent studies have looked at the contemporary prevalence of DPN in a large cohort of adults with type 1 diabetes in the post-DCCT era reflecting contemporary practices of clinical care. Using the MNSIQ, a survey tool previously found to be reliable for assessment of DPN symptoms in patients with type 1 diabetes, we found a prevalence of 11%, which is lower than prior reported prevalence rates for similar diabetes duration, including the DCCT/EDIC cohort. This may be related to the younger age of the cohortg with type 1 diabetes in the T1D Exchange Clinic Registry or to the lower sensitivity of this screening instrument, considering that DPN in the DCCT and EDIC studies was defined using either a comprehensive outcome that included symptoms, clinical signs as documented by a board-certified neurologist and electrodiagnostic criteria in several peripheral nerves, or the entire MNSI that included the questionnaire and the clinical examination (9). The more recent SEARCH for Diabetes in Youth Study, evaluating the prevalence and risk factors for DPN in youth and adolescents with type 1 diabetes, found a DPN prevalence of 7% in participants with an average age of 21 years, which is in line with our age-stratified DPN prevalence of 4% in 18–25-year-olds (18).
Similar to DCCT/EDIC or other prior observational cohorts, including the Pittsburgh EDC and the EURODIAB Prospective Complications studies, we found that DPN prevalence rates were associated with traditional risk factors including age, duration of diabetes, and glucose control as documented by glycosylated hemoglobin (8,9,16). Additionally, we found associations between DPN and several CVD risk factors, including higher BMI, triglycerides, and cigarette use as well as a clear association between CVD and DPN. The role of traditional CVD risk factors, including higher BMI, triglycerides, and smoking, in the development of DPN in people with type 1 diabetes was first observed in the EURODIAB cohort (19). In later studies, including in cohorts with type 1 diabetes and available sural nerve biopsy specimens, higher triglycerides in particular were shown to be associated with rapid DPN progression as documented by loss of sural nerve myelinated fibers, independent of other DPN risk factors including age, glycemic control, and duration (20). Higher BMI, weight and waist circumference play a pivotal role as risk factors for DPN in patients with metabolic syndrome, prediabetes, and type 2 diabetes as well (18,21). In contrast to the findings in EURODIAB, the Pittsburgh EDC, and SEARCH, we found no association between other lipid parameters and DPN in this cohort (8,18,19). Similarly, we found no associations between systolic or diastolic blood pressure and DPN, although prior studies of cohorts with type 1 diabetes have found associations between hypertension and DPN (8,18,19). Not unexpectedly, we also found associations between DPN and other microvascular complications, including retinopathy and Charcot arthropathy, which is also in concert with the findings reported by Tesfaye et al. (16) in the EURODIAB cohort.
Although the associations of DPN with the more traditional risk factors described above was expected, this study unveiled several important new findings. The first is a higher prevalence (68%) of painful DPN in this cohort. This is in contrast with prior reports showing in general only up 20–30% of DPN is painful (1,22,23). However, this relatively higher prevalence of painful DPN in this cohort could be due to bias associated with both the self-selection of participants involved in our study as well as the instrument used to diagnose DPN, given that the foot burning pain question is one of the four questions used in outcome definition. As expected, medication use for neuropathic pain was common in this cohort, with 29% of MNSIQ-defined DPN participants using at least one agent for pain.
The next interesting finding is a slightly higher prevalence of DPN in women, irrespective of pain, which has not been found in other large cohorts with type 1 diabetes (8,16,18,24). However, there was a higher rate of responders among women with type 1 diabetes in this survey, which may have contributed. Somewhat unexpectedly, we did not find a female sex predominance of painful DPN. Higher prevalence of pain in general was described in women in many disease states, including in diabetes (25), and prior studies report that women with diabetes experience more severe pain despite milder nerve injury (26). Our findings are also in contrast to prior reports in cohorts with diabetes in the U.K. and Canada (27,28).
Another important finding that has emerged is the association between DPN and acute diabetes complications, including both SH and DKA events within the past 3 months. This finding highlights a potential relationship between DPN and glycemic variability, which was suggested by prior findings in smaller cohorts (29,30), although not documented in DCCT/EDIC (31). However, glucose variability in the DCCT/EDIC study was derived from the 7-point glucose profile, because no CGM data were available. A more recent study evaluating CGM-derived indices of glycemic variability in relationship to DPN as measured by nerve conduction studies demonstrated that the mean amplitude of glycemic excursions was strongly associated with medial plantar neuropathy (32). Larger observational studies with more stringent measures of glycemic variability are needed to confirm these observations. Interestingly, participants with DPN were also more likely to have gastroparesis. Although correlations DPN and forms of autonomic neuropathy, including gastroparesis, may reflect a more advanced disease associated with both small-and large-fiber dysfunction, fluctuations in blood glucose levels in both hypo- and hyperglycemic range have been shown to directly affect gastric emptying (33), potentially explaining these findings.
Furthermore, here we report associations between elements of socioeconomic status and DPN. Not surprisingly, we found that DPN prevalence was associated with markers of lower socioeconomic status, including lower education levels and more reliance on public insurance options. The impact of the social determinants on diabetes self-management and glycemic control in diabetes is emerging as an important theme, particularly in the U.S., due to the rising costs of insulin and the resulting deliberate underuse of insulin (34). The association between the social determinants of health and markers of microvascular and cardiovascular complications has been explored in pediatric poulations with type 1 diabetes and in a Scottish cohort of adults with type 1 diabetes, and there appears to be an adverse relationship (35–37); however, this topic needs further exploration in the adult population with type 1 diabetes in the U.S. The prospective German Diabetes Study, which aims to evaluate nonglycemic risk factors for progression of complications, including socioeconomic and psychosocial factors, may provide more clarity in the future (38).
Our study has several limitations. The first is that the T1D Exchange is not a true population-based cohort of all patients with type 1 diabetes, a limitation that has been well-described in prior publications (11), although >63 U.S. diabetes clinics, both academic and private, are represented in this current study, reflective of contemporary diabetes care practices across the U.S. This has the potential to underestimate the MSNIQ-defined DPN prevalence found in these analyses because certain demographics may not be adequately represented. This study, for example, found that the lack of private insurance is associated with MNSIQ-defined DPN; however, 80% of this survey’s participants had private insurance, which is higher than other cohorts of patients with type 1 diabetes that reported insurance information (39,40). Furthermore, the MNSIQ survey responders were self-selected to participate in this study, which also limits the generalizability of our findings. However, even though estimated frequencies and prevalence of MNSIQ-defined DPN and various factors could be over- or underestimates, this is unlikely to affect the interpretation of associations between one variable and another (11).
Another limitation is the measurement of DPN in this cohort. While the MNSIQ has been found to be a reliable screening method for DPN in DCCT/EDIC and other large cohorts, prior studies reported only 40% sensitivity for the ≥4 threshold on the survey for detecting DPN (13). Indeed, based on a sensitivity analysis using cutoffs of ≥2 and ≥3 on the MNSIQ, the prevalence may be as high as 17–39%; however, the number of false positives likely also rises. Furthermore, being a symptom-based questionnaire, it is possible that we missed individuals with DPN with less severe neuropathy symptoms or those who may present with clinical findings in the absence of any symptoms that would have been picked up with the examination portion of the full MNSI instrument. This is the rationale behind referring to MNSI-defined DPN as MNSI questionnaire or “MNSIQ-defined DPN,” although it may be more appropriate to call these participants “symptomatic DPN” participants (13). In terms of classification of painful and painless DPN, the symptom-based MSNIQ questionnaire also has the ability to overestimate painful neuropathy, which may be contributing to the high prevalence of painful DPN in this cohort. Although we found that the use of several medications, including duloxetine, pregabalin, gabapentin, or tricyclic antidepressants commonly prescribed for neuropathic pain, was high in MNSIQ-defined DPN participants, we recognize that these agents may be used for a variety of different conditions, including treatment of depression or seizure disorders, so the frequency of use of these agents may be overestimated in our cohort.
Finally, this current study is cross-sectional, and therefore, we cannot assume any causality for our associations between the risk factors reported and the presence of DPN. Nonetheless, this study of >5,900 participants is the largest study to date to evaluate the prevalence of DPN in type 1 diabetes.
In summary, in this large cohort with type 1 diabetes, a DPN prevalence of 11% is markedly lower compared with prior published studies in earlier cohorts, suggesting potential beneficial effects of the improvement in the clinical care and risk factor control in the U.S. adult population with type 1 diabetes. Although we confirmed expected associations between DPN and traditional glycemic and vascular risk factors, we have also found associations with novel nonglycemic DPN risk factors, such as CVD risk factors, both SH and DKA, potentially reflecting effects of glycemic variability, and lower socioeconomic status, highlighting the importance of social determinants of health in patients with type 1 diabetes. This emphasizes the importance of ongoing research for more disadvantaged populations with type 1 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors would like to thank all participants and clinicians who contributed to the T1D Exchange Clinic Registry.
Funding. Funding for the T1D Exchange was provided by the Leona M. and Harry B. Helmsley Charitable Trust (grant G-2016PG-T1D053). R.P.-B. was also supported by grants R01-DK-107956-01 and U01-DK-119083 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.R.M.-S. and R.P.-B. designed the study, researched data, contributed to data interpretation, and wrote and edited the manuscript. Z.L. and N.C.F. contributed to data interpretation, performed statistical analysis, and wrote and edited the manuscript. V.S., G.A., J.B.M., R.P., E.T., and L.A. contributed to data interpretation and edited and revised the manuscript. N.C.F. is the guarantor of this work, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1583/-/DC1.
Contributor Information
Collaborators: T1D Exchange Clinic Networ k, Ruth Weinstock, Roberto Izquierdo, Umair Sheikh, Patricia Conboy, Jane Bulger, Suzan Bzdick, Georgeanna Klingensmith, Carolyn Banion, Jennifer Barker, Cindy Cain, Kristen Nadeau, Marian Rewers, Arleta Rewers, Robert Slover, Andrea Steck, Paul Wadwa, Philip Zeitler, Guy Alonso, Greg Forlenza, Andrea Gerard-Gonzalez, Melanie Green, Susan Gross, Shideh Majidi, Laurel Messer, Tyler Reznick-Lipina, Emily Simmons, Katie Thivener, Isabel Weber, Steven Willi, Terri Lipman, Olena Kucheruk, Pantea Minnock, Cathy Carchidi, Brian Grant, Diana Olivos, Linda DiMeglio, Tamara Hannon, Carmella Evans-Molina, David Hansen, Tina Pottorff, Stephanie Woerner, Megan Hildinger, Robin Hufferd, America Newnum, Devyn Purtlebaugh, Lisa Smith, Kathleen Wendholt, Robin Goland, Rachelle Gandica, Kristen Williams, Sarah Pollack, Emily Casciano, Julia Hochberg, Cecilia Uche, Joyce Lee, Brigid Gregg, Meng Tan, Lynn Ang, Rodica Pop-Busui, Inas Thomas, Emily Dhadphale, Janet Dominowski, Ashley Garrity, Virginia Leone, Cynthia Plunkett, Brittany Plunkett, Roshanak Monzavi, Clement Cheung, Lynda Fisher, Mimi Kim, Brian Miyazaki, Pisit Pitukcheewanont, Anna Sandstrom, Juliana Austin, Nancy Change, Jennifer Raymond, Brian Ichihara, Megan Lipton, Jaquelin Flores Garcia, Satish Garg, Aaron Michels, Rachel Garcetti, Raymond Gutin, Sarit Polsky, Viral Shah, Mary Voelmle, Lisa Myers, Gregory Coe, Jamie Demmitt, Yesenia Garcia Reyes, Dominique Giordano, Prakriti Joshee, Emily Nease, Nhung Nguyen, Joseph Wolfsdorf, Maryanne Quinn, Constance Fontanet, Susmita Mukherjee, Kathleen Bethin, Teresa Quattrin, Indrajit Majumdar, Lucy Mastrandrea, Emily Gorman, Amanda House, Sharon Michalovic, Wanda Musial, Barbara Shine, Andrew Ahmann, Jessica Castle, Farahnaz Joarder, Diana Aby-Daniel, Ines Guttmann-Bauman, Bethany Klopfenstein, Victoria Morimoto, Nancy Cady, Rebecca Fitch, Donald DeFrang, Kristin Jahnke, Claire Patoine, Vandana Raman, Carol Foster, Mary Murray, Trina Brown, Cassandra Davis, Hillarie Slater, Jennifer Langvardt, Bruce Bode, Jennifer Boyd, Joseph Johnson, Christopher Newton, Jonathan Ownby, RaShonda Hosey, Nitin Rastogi, Blake Winslett, Irl Hirsch, Anthony DeSantis, R. Alan Failor, Carla Greenbaum, Dace Trence, Subbulaxmi Trikudanathan, Dori Khakpour, Pam Thomson, Lori Sameshima, Corinna Tordillos, Mark Clements, Angela Turpin, Ghufran Babar, Julia Broussard, Joe Cernich, Kavitha Dileepan, Max Feldt, Wayne Moore, Tiffany Musick, Susana Patton, Yun Yan, Sarah Tsai, Jennifer Bedard, Aliza Elrod, Lois Hester, Marissa Beidelschies, Julie de la Garza, Emily Haith, Jennifer James, Elizabeth Ramey, Jessica Slover, Armenthia Valentine, Darta Watkins, Misty Whisenhunt, Jami Wierson, Darrell Wilson, Bruce Buckingham, David Maahs, Priya Prahalad, Liana Hsu, Ryan Kingman, Ideen Tabatabai, David Liljenquist, Mark Sulik, Carl Vance, Jean Halford, Christine Funke, Yaw Appiagyei-Dankah, Emily Beltz, Karen Moran, Harold Starkman, Barbara Cerame, Daisy Chin, Laurie Ebner-Lyon, Kristen Sabanosh, Lawrence Silverman, Christine Wagner, Sunita Cheruvu, Marie Fox, Frances Melchionne, Richard Bergenstal, Marcia Madden, Thomas Martens, Amy Criego, Margaret Powers, Anders Carlson, Shannon Beasley, Beth Olson, LeeAnn Thomas, Kathleen McCann, Sean Dunnigan, Charlotte Ashanti, Jill Simmons, William Russell, Sarah Jaser, Jennifer Kelley, Faith Brendle, Lauren Williams, Kimberly Savin, Kimberly Flowers, George Williams, Emily Hamburger, Angelia Davis, Brenna Hammel, Eda Cengiz, William Tamborlane, Kate Weyman, Michelle Van Name, Neha Patel, Jennifer Sherr, Eileen Tichy, Amy Steffen, Melinda Zgorski, Lori Carria, Jennifer Finnegan, Elvira Duran, Sanjeev Mehta, Michelle Katz, Lori Laffel, Elisa Giani, Rebecca Snelgrove, Anat Hanono, Persis Commissariat, Julie Griffith, Ashley Atkins, Kara Harrington, Kenny Kim, Luisa Masclans, Nisha Naik, Louise Ambler-Osborn, Alan Schultz, Charlotte Cohen, Brittany Anderson, Janet McGill, Andrea Granados, Mary Jane Clifton, Stacy Hurst, Sarah Kissel, Carol Recklein, Davida Kruger, Arti Bhan, Terri Brown, Andreana Tassopoulos, Angela Hailey, Heather Remtema, Terra Cushman, Kupper Wintergerst, Sara Watson, Suzanne Kingery, Lauren Rayborn, Heather Rush, Michael Foster, Amy Deuser, Manuel Rodriguez-Luna, Stephanie Eubanks, Henry Rodriguez, Sureka Bollepalli, Laura Smith, Dorothy Shulman, E. Verena Jorgensen, Emily Eyth, Rachel Brownstein, Janet Rodriguez, Juanita O’Brian, Grazia Aleppo-Kacmarek, Allison Hahr, Mark Molitch, El Muayed, Daniel Toft, Candice Fulkerson, Daphne Adelman, Elaine Massaro, Kimberly Webb, Anne Peters, Valerie Ruelas, Mark Harmel, Mark Daniels, Nikta Forghani, Timothy Flannery, Christina Reh, Amrit Bhangoo, Himala Kashmiri, Keirsten Montgomery, Lien Trinh, Heather Speer, Kristen Lane, Cassie Miller, Christine Burt Solorzano, Jennifer Puskaric, Robert Benjamin, Deanna Adkins, Amber Spruill, Cathy Williams, Eva Tsalikian, Michael Tansey, Nidhi Bansl, Joanne Cabbage, Julie Coffey, Rachel Bisbee, Desmond Schatz, Michael Clare-Salzler, Kenneth Cusi, Becky Fudge, Mike Haller, Collette Meehan, Henry Rohrs, Janet Silverstein, Ashby Walker, Anastasia Albanese-O’Niell, Stephanie Foss, Janey Adams, Miriam Cintron, Nicole Thomas, Michael Gottschalk, Ron Newfield, Marla Hashiguchi, David Sparling, Jeanie Tryggested, Joni Beck, Joane Less, Linda Weber, Saleh Adi, Stephen Gitelman, Srinath Sanda, Jenise Wong, Mary McDonnell, Monica Mueller, Zara Izadi, Swaroop Mistry, Bryce Nelson, Lisa Looper, Carrie Frost, Maria Redondo, Sarah Lyons, Sara Klinepeter, Kelly Fegan-Bohm, Fida Bacha, Daniel DeSalvo, Ashley Butler, Marisa Hilliard, Farida Khetani, Ronald Yulatic, Robert Hudson, Laura Irvine, Sadia Zubair, Cory Pace, Abelin Pitrello, Wendy Levy, Charity Njoku, William Zipf, Jennifer Dyer, Rolando Lozano, Diane Seiple, Grant Corven, Megan Jaycox, Jamie Wood, Sarah Macleish, Rose Gubitosi-Klug, Ramon Adams, Paul McGuigan, Terri Casey, Wendy Campbell, Julie Kittelsrud, Ashutosh Gupta, Vikki Peterson, Ingrid Libman, Ana Diaz, David Jelley, Christina Crowder, Dana Greer, Julia Crawford, Sharnella Goudeau, Catherine Pihoker, Joyce Yi-Frazier, Susan Kearns, Michael Pascual, Beth Loots, Natalie Beauregard, Michael Rickels, Shannon O’Brien, Shivani Agarwal, Amy Peleckis, Cornelia Dalton-Bakes, Eileen Markmann, Guillermo Umpierrez, Andrew Muir, Clementina Ramos, Keywan Behbahani, Neil Dhruv, Nolan Gartzman, Brandon Nathan, Melena Bellin, Muna Sunni, Nancy Flaherty, Janice Leschyshyn, Kara Schmid, Darcy Weingartner, Marrissa Ludwig, Brittney Nelson, Anne Kogler, Avery Bartyzal, Anne Street, Beth Pappenfus, Jessica Sweet, John Buse, Laura Young, Katherine Bergamo, April Goley, Marian Kirkman, Jamie Diner, Alex Kass, Milana Dezube, Kathleen Arnold, Traci Evans, Sharon Sellers, Scott Blackman, Kimber-Lee Abel, Lisa Rasbach, Omar Ali, Peter Wolfgram, Rosanna Fiallo-Sharer, Joanna Kramer, Christina Beesley, Clare Bingham-Tyson, Rachel Unteutsh, David Harlan, Mary Lee, Leslie Soyka, Penny Feldman, Michael Thompson, Karen Gallagher-Dorval, Lisa Hubacz, Celia Hartigan, Carol Ciccarelli, Rachel Edelen, Michelle Edelen, Trista Borgwadt, Kirstin Stauffacher, Kelly DeGrote, Crystal Gruetzmacher, Michael Shepperd, Anuj Bhargava, Diana Wright, Kathleen Fitzgerald, Teck Khoo, Natalie Young, Lisa Borg, Kirstie Stifel, Cindy Rail, Luis Casas, Elizabeth Eidenshink, Christina Huber, Alex Rieder, Amy Tuchscherer, Megan Broadbent, Lawrence Dolan, Sarah Corathers, Jessica Kichler, Nicole Sheanon, Holly Baugh, Debbie Standiford, Tammy Weis, Catherine Fox, Carrie Schultz, Amy Ritter, Francesco Vendrame, Carlos Blashke, Della Matheson, Natalia Sanders-Branca, Justen Rudolph, Doris Biersdorf, Jane Fitch-Danielson, Dara Eckerle-Mize, Janet Fry, Dianne Davis, Cynthia Lovell, Robert Hoffman, Monika Chaudhari, Manmohan Kamboj, Lindsey Carr, Julie Blehm, Anthony Tello, Julie Ann Walter, Rhonda Ward, Gabriel Blomquist, Maria Stewart, Paige Cross, Sarah Racki, Lindsey Sterchi, Diane Gouine, Becky Kiesow, Stephanie Welch, Athena Philis-Tsimikas, George Daily, Amy Chang, James McCallum, Isabel Garcia, Teresa Vela, Ioanna Loupasi, Rosario Rosal, Elena Toschi, Roeland Middelbeek, Medha Munshi, Christine Slyne, Astrid Atakov-Castillo, Larry Fox, Nelly Mauras, Rachel Wasserman, Ligeia Damaso, Kim Englert, Kaitlin Sikes, Kim Ponthieux, Louis Phillipson, Ashley Cohen, Gail Gannon, Larry Deeb, April Shiver, Leroy Schroeder, Wendi Schworm, Kristina Graham, Carol Levy, David Lam, Elizabeth Burtman, Camilla Levister, Selassie Ogyaadu, Heidi Gassner, Julie Duke, Leslie Touger, Dorothee Newbern, Francine Hoekstra, Katerina Harwood, Vijaya Prasad, JoAnne Daguanno, Richard Pratley, Karen Corbin, Mia Wright, Susann Nagel, Natasha Water, Matthew Ghere, Keri Whitaker, Rubina Heptulla, Ranjitha Katikaneni, Doreen Johnson-Newell, Jill Crandall, Danielle Powell, Valentin Anghel, Steven Ghanny, Javier Aisenberg, Amy Chartoff, Jennifer Sivitz, Susan Mathus, Toni-Lyn Cospito, Kathryn Thailkill, John Fowlkes, Evangelia Kalaitzoglou, Alba Morales Pozzo, and Kathy Edwards
References
- 1.Pop-Busui R, Boulton AJ, Feldman EL, et al. . Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–2375 [DOI] [PubMed] [Google Scholar]
- 3.Schmidt BM, Holmes CM, Ye W, Pop-Busui R. A tale of two eras: mining big data from electronic health records to determine limb salvage rates with podiatry. Curr Diabetes Rev 2019;15:497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustapa A, Justine M, Mohd Mustafah N, Jamil N, Manaf H. Postural control and gait performance in the diabetic peripheral neuropathy: a systematic review. BioMed Res Int 2016;2016:9305025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perazzolo M, Reeves ND, Bowling FL, Boulton AJM, Raffi M, Marple-Horvat DE. Altered accelerator pedal control in a driving simulator in people with diabetic peripheral neuropathy. Diabet Med. 29 March 2019 [Epub ahead of print]. DOI: 10.1111/dme.13957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgnakke WS, Anderson PF, Shannon C, Jivanescu A. Is there a relationship between oral health and diabetic neuropathy? Curr Diab Rep 2015;15:93. [DOI] [PubMed] [Google Scholar]
- 7.Veresiu AI, Bondor CI, Florea B, Vinik EJ, Vinik AI, Gâvan NA. Detection of undisclosed neuropathy and assessment of its impact on quality of life: a survey in 25,000 Romanian patients with diabetes. J Diabetes Complications 2015;29:644–649 [DOI] [PubMed] [Google Scholar]
- 8.Maser RE, Steenkiste AR, Dorman JS, et al. . Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989;38:1456–1461 [DOI] [PubMed] [Google Scholar]
- 9.Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group . Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes–2019. Diabetes Care 2019;42(Suppl. 1):S124–S138 [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network . The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 12.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 13.Herman WH, Pop-Busui R, Braffett BH, et al.; DCCT/EDIC Research Group . Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini YKA, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006;93:491–507 [Google Scholar]
- 16.Tesfaye S, Stevens LK, Stephenson JM, et al. . Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 1996;39:1377–1384 [DOI] [PubMed] [Google Scholar]
- 17.Christensen MMB, Hommel EE, Jørgensen ME, von Scholten BJ, Fleischer J, Hansen CS. Prevalence of diabetic neuropathy in young adults with type 1 diabetes and the association with insulin pump therapy. Diabetes Technol Ther. 21 November 2018 DOI: 10.1089/dia.2018.0249 [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal M, Divers J, Dabelea D, et al. . Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for Diabetes in Youth study. Diabetes Care 2017;40:1226–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tesfaye S, Chaturvedi N, Eaton SE, et al.; EURODIAB Prospective Complications Study Group . Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 20.Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009;58:1634–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callaghan BC, Gao L, Li Y, et al. . Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol 2018;5:397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–1522 [DOI] [PubMed] [Google Scholar]
- 23.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group . Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10:393–400 [DOI] [PubMed] [Google Scholar]
- 24.Albers JW, Herman WH, Pop-Busui R, et al.; Diabetes Control and Complications Trial /Epidemiology of Diabetes Interventions and Complications Research Group . Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010;33:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, Bril V. Sex differences in neuropathic pain intensity in diabetes. J Neurol Sci 2018;388:103–106 [DOI] [PubMed] [Google Scholar]
- 27.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011;34:2220–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardinez N, Lovblom LE, Bai JW, et al. . Sex differences in neuropathic pain in longstanding diabetes: results from the Canadian Study of Longevity in Type 1 Diabetes. J Diabetes Complications 2018;32:660–664 [DOI] [PubMed] [Google Scholar]
- 29.Jaiswal M, McKeon K, Comment N, et al. . Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care 2014;37:2616–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stem MS, Dunbar GE, Jackson GR, Farsiu S, Pop-Busui R, Gardner TW. Glucose variability and inner retinal sensory neuropathy in persons with type 1 diabetes mellitus. Eye (Lond) 2016;30:825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachin JM, Bebu I, Bergenstal RM, et al.; DCCT/EDIC Research Group . Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the Diabetes Control and Complications Trial. Diabetes Care 2017;40:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akaza M, Akaza I, Kanouchi T, Sasano T, Sumi Y, Yokota T. Nerve conduction study of the association between glycemic variability and diabetes neuropathy. Diabetol Metab Syndr 2018;10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plummer MP, Jones KL, Cousins CE, et al. . Hyperglycemia potentiates the slowing of gastric emptying induced by exogenous GLP-1. Diabetes Care 2015;38:1123–1129 [DOI] [PubMed] [Google Scholar]
- 34.Herkert D, Vijayakumar P, Luo J, et al. . Cost-related insulin underuse among patients with diabetes. JAMA Intern Med 2019;179:112–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inman M, Daneman D, Curtis J, et al. . Social determinants of health are associated with modifiable risk factors for cardiovascular disease and vascular function in pediatric type 1 diabetes. J Pediatr 2016;177:167–172 [DOI] [PubMed] [Google Scholar]
- 36.Cummings LAM, Clarke A, Sochett E, et al. . Social determinants of health are associated with markers of renal injury in adolescents with type 1 diabetes. J Pediatr 2018;198:247–253.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collier A, Ghosh S, Hair M, Waugh N. Gender differences and patterns of cardiovascular risk factors in Type 1 and Type 2 diabetes: a population-based analysis from a Scottish region. Diabet Med 2015;32:42–46 [DOI] [PubMed] [Google Scholar]
- 38.Szendroedi J, Saxena A, Weber KS, et al.; GDS Group . Cohort profile: the German Diabetes Study (GDS). Cardiovasc Diabetol 2016;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everett E, Mathioudakis NN. Association of socioeconomic status and DKA readmission in adults with type 1 diabetes: analysis of the US National Readmission Database. BMJ Open Diabetes Res Care 2019;7:e000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder LL, Stafford JM, Dabelea D, et al. . Socio-economic, demographic, and clinical correlates of poor glycaemic control within insulin regimens among children with Type 1 diabetes: the SEARCH for Diabetes in Youth Study. Diabet Med 2019;36:1028–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.